MgrB-Dependent Colistin Resistance in Klebsiella pneumoniae Is Associated with an Increase in Host-to-Host Transmission
- PMID: 35311534
- PMCID: PMC9040857
- DOI: 10.1128/mbio.03595-21
MgrB-Dependent Colistin Resistance in Klebsiella pneumoniae Is Associated with an Increase in Host-to-Host Transmission
Abstract
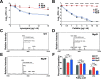
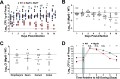
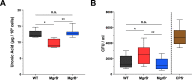
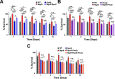
Due to its high transmissibility, Klebsiella pneumoniae is one of the leading causes of nosocomial infections. Here, we studied the biological cost of colistin resistance, an antibiotic of last resort, in this opportunistic pathogen using a murine model of gut colonization and transmission. Colistin resistance in K. pneumoniae is commonly the result of the inactivation of the small regulatory protein MgrB. Without a functional MgrB, the two-component system PhoPQ is constitutively active, leading to an increase in lipid A modifications and subsequent colistin resistance. Using an isogenic mgrB deletion mutant (MgrB-), we demonstrate that the mutant's colistin resistance is not associated with a fitness defect under in vitro growth conditions. However, in our murine model of K. pneumoniae gastrointestinal (GI) colonization, the MgrB- colonizes the gut poorly, allowing us to identify a fitness cost. Moreover, the MgrB- mutant has higher survival outside the host compared with the parental strain. We attribute this enhanced survivability to dysregulation of the PhoPQ two-component system and accumulation of the master stress regulator RpoS. The enhanced survival of MgrB- may be critical for its rapid host-to-host transmission observed in our model. Together, our data using multiple clinical isolates demonstrate that MgrB-dependent colistin resistance in K. pneumoniae comes with a biological cost in gut colonization. However, this cost is mitigated by enhanced survival outside the host and consequently increases its host-to-host transmission. Additionally, it underscores the importance of considering the entire life cycle of a pathogen to determine the actual biological cost associated with antibiotic resistance. IMPORTANCE The biological cost associated with colistin resistance in Klebsiella pneumoniae was examined using a murine model of K. pneumoniae gut colonization and fecal-oral transmission. A common mutation resulting in colistin resistance in K. pneumoniae is a loss-of-function mutation of the small regulatory protein MgrB that regulates the two-component system PhoPQ. Even though colistin resistance in K. pneumoniae comes with a fitness defect in gut colonization, it increases bacterial survival outside the host enabling it to transmit more effectively to a new host. The enhanced survival is dependent upon the accumulation of RpoS and dysregulation of the PhoPQ. Hence, our study expands our understanding of the underlying molecular mechanism contributing to the transmission of colistin-resistant K. pneumoniae.
Keywords: antimicrobial peptides; gastrointestinal infection; host-to-host transmission; infection control; stress adaptation; two-component regulatory systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
The cost of resistance.Nat Rev Microbiol. 2022 Jun;20(6):317. doi: 10.1038/s41579-022-00733-w. Nat Rev Microbiol. 2022. PMID: 35365811 No abstract available.
Similar articles
-
Global prevalence of mutation in the mgrB gene among clinical isolates of colistin-resistant Klebsiella pneumoniae: a systematic review and meta-analysis.Front Microbiol. 2024 Jun 7;15:1386478. doi: 10.3389/fmicb.2024.1386478. eCollection 2024. Front Microbiol. 2024. PMID: 38912352 Free PMC article.
-
The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae.J Antimicrob Chemother. 2015 Jan;70(1):75-80. doi: 10.1093/jac/dku323. Epub 2014 Sep 3. J Antimicrob Chemother. 2015. PMID: 25190723
-
A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence.EMBO Mol Med. 2017 Apr;9(4):430-447. doi: 10.15252/emmm.201607336. EMBO Mol Med. 2017. PMID: 28202493 Free PMC article.
-
In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator.Antimicrob Agents Chemother. 2013 Nov;57(11):5521-6. doi: 10.1128/AAC.01480-13. Epub 2013 Aug 26. Antimicrob Agents Chemother. 2013. PMID: 23979739 Free PMC article.
-
MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae.Cells. 2022 Sep 26;11(19):2995. doi: 10.3390/cells11192995. Cells. 2022. PMID: 36230959 Free PMC article. Review.
Cited by
-
The cost of resistance.Nat Rev Microbiol. 2022 Jun;20(6):317. doi: 10.1038/s41579-022-00733-w. Nat Rev Microbiol. 2022. PMID: 35365811 No abstract available.
-
Role of the ISKpn element in mediating mgrB gene mutations in ST11 hypervirulent colistin-resistant Klebsiella pneumoniae.Front Microbiol. 2023 Sep 28;14:1277320. doi: 10.3389/fmicb.2023.1277320. eCollection 2023. Front Microbiol. 2023. PMID: 37840706 Free PMC article.
-
From Farm to Fork: Persistence of Clinically Relevant Multidrug-Resistant and Copper-Tolerant Klebsiella pneumoniae Long after Colistin Withdrawal in Poultry Production.Microbiol Spectr. 2023 Aug 17;11(4):e0138623. doi: 10.1128/spectrum.01386-23. Epub 2023 Jul 10. Microbiol Spectr. 2023. PMID: 37428073 Free PMC article.
-
Phylogenetic context of antibiotic resistance provides insights into the dynamics of resistance emergence and spread.medRxiv [Preprint]. 2025 Jun 5:2025.06.04.25328982. doi: 10.1101/2025.06.04.25328982. medRxiv. 2025. PMID: 40502560 Free PMC article. Preprint.
-
Global prevalence of mutation in the mgrB gene among clinical isolates of colistin-resistant Klebsiella pneumoniae: a systematic review and meta-analysis.Front Microbiol. 2024 Jun 7;15:1386478. doi: 10.3389/fmicb.2024.1386478. eCollection 2024. Front Microbiol. 2024. PMID: 38912352 Free PMC article.
References
-
- World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2017. Global Antimicrobial Resistance Surveillance System (GLASS) report: early Implementation (2016-2017). World Health Organization, Geneva, Switzerland.
-
- European Centre for Disease Prevention and Control. 2013. Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. European Centre for Disease Prevention and Control, Stockholm, Sweden.
-
- Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto F, Cristini F, Trapani F, Gaibani P, Viale P. 2014. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore 93:298–309. doi:10.1097/MD.0000000000000111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
