Murine Respiratory Tract Infection with Classical Klebsiella pneumoniae Induces Bronchus-Associated Lymphoid Tissue
- PMID: 35311545
- PMCID: PMC9022520
- DOI: 10.1128/iai.00596-21
Murine Respiratory Tract Infection with Classical Klebsiella pneumoniae Induces Bronchus-Associated Lymphoid Tissue
Abstract
Klebsiella pneumoniae is a Gram-negative, opportunistic pathogen that commonly causes nosocomial pneumonia, urinary tract infection, and septicemia. Our recent work utilizing a murine model of respiratory tract infection with classical K. pneumoniae demonstrated leukocyte aggregates in the lungs of mice at 28 days postinfection. Here, we sought to characterize the composition and development of these structures. Histopathological analyses of murine lungs revealed immune cell clusters surrounding the pulmonary vasculature and airways by 14 days postinfection, resembling inducible bronchus-associated lymphoid tissue (iBALT). Further investigation of these structures demonstrated central B cell aggregates with concomitant dispersed T cells. At day 28 postinfection, these lymphoid clusters expressed germinal center markers and CXCL12, qualifying these structures as iBALT with nonclassical B cell follicles. Investigations in mutant mice revealed that those lacking B and/or T cells were not able to form fully defined iBALT structures, although some rudimentary B cell clusters were identified in mice lacking T cells. The longevity of K. pneumoniae-induced BALT was assessed for up to 120 days postinfection. Lymphoid aggregates significantly decreased in size and quantity by 90 days after K. pneumoniae infection; however, aggregates persisted in mice that were restimulated with K. pneumoniae every 30 days. Finally, infections of mice with an array of classical K. pneumoniae clinical isolates demonstrated that the development of these structures is a common feature of K. pneumoniae lung infection. Together, these data confirm that murine lungs infected with K. pneumoniae develop iBALT, which may play a role in pulmonary immunity to this troublesome pathogen.
Keywords: B cells; Klebsiella pneumoniae; T cells; iBALT; induced bronchus-associated lymphoid tissue; pneumonia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

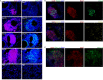

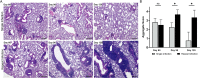

Similar articles
-
A murine model demonstrates capsule-independent adaptive immune protection in survivors of Klebsiella pneumoniae respiratory tract infection.Dis Model Mech. 2020 Mar 26;13(3):dmm043240. doi: 10.1242/dmm.043240. Dis Model Mech. 2020. PMID: 32298236 Free PMC article.
-
A Porcine Ex Vivo Lung Perfusion Model To Investigate Bacterial Pathogenesis.mBio. 2019 Dec 3;10(6):e02802-19. doi: 10.1128/mBio.02802-19. mBio. 2019. PMID: 31796543 Free PMC article.
-
Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis.J Clin Invest. 2006 Dec;116(12):3183-94. doi: 10.1172/JCI28756. J Clin Invest. 2006. PMID: 17143328 Free PMC article.
-
Regulation of inducible BALT formation and contribution to immunity and pathology.Mucosal Immunol. 2010 Nov;3(6):537-44. doi: 10.1038/mi.2010.52. Epub 2010 Sep 1. Mucosal Immunol. 2010. PMID: 20811344 Review.
-
Bronchus-associated lymphoid tissue (BALT) structure and function.Adv Immunol. 2010;107:187-241. doi: 10.1016/B978-0-12-381300-8.00007-1. Adv Immunol. 2010. PMID: 21034975 Free PMC article. Review.
Cited by
-
Capsular polysaccharide inhibits vaccine-induced O-antigen antibody binding and function across both classical and hypervirulent K2:O1 strains of Klebsiella pneumoniae.PLoS Pathog. 2023 May 5;19(5):e1011367. doi: 10.1371/journal.ppat.1011367. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37146068 Free PMC article.
-
Inflammation, tertiary lymphoid structures, and lung cancer: a bibliometric analysis.Transl Lung Cancer Res. 2024 Oct 31;13(10):2636-2648. doi: 10.21037/tlcr-24-350. Epub 2024 Oct 28. Transl Lung Cancer Res. 2024. PMID: 39507023 Free PMC article.
-
Bordetella spp. block eosinophil recruitment to suppress the generation of early mucosal protection.Cell Rep. 2023 Nov 28;42(11):113294. doi: 10.1016/j.celrep.2023.113294. Epub 2023 Oct 25. Cell Rep. 2023. PMID: 37883230 Free PMC article.
-
Lung infection with classical Klebsiella pneumoniae strains establishes robust macrophage-dependent protection against heterologous reinfection.Microbes Infect. 2024 Nov-Dec;26(8):105369. doi: 10.1016/j.micinf.2024.105369. Epub 2024 May 28. Microbes Infect. 2024. PMID: 38815803 Free PMC article.
-
The Derivative Difluoroboranyl-Fluoroquinolone "7a" Generates Effective Inhibition Against the S. aureus Strain in a Murine Model of Acute Pneumonia.Curr Issues Mol Biol. 2025 Feb 10;47(2):110. doi: 10.3390/cimb47020110. Curr Issues Mol Biol. 2025. PMID: 39996831 Free PMC article.
References
-
- Dhesi Z, Enne VI, Brealey D, Livermore DM, High J, Russell C, Colles A, Kandil H, Mack D, Martin D, Page V, Parker R, Roulston K, Singh S, Wey E, Swart AM, Stirling S, Barber JA, O’Grady J, Gant V. 2020. Organisms causing secondary pneumonias in COVID-19 patients at 5 UK ICUs as detected with the FilmArray test. medRxiv. 10.1101/2020.06.22.20131573. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

