Biofilm Maintenance as an Active Process: Evidence that Biofilms Work Hard to Stay Put
- PMID: 35311557
- PMCID: PMC9017327
- DOI: 10.1128/jb.00587-21
Biofilm Maintenance as an Active Process: Evidence that Biofilms Work Hard to Stay Put
Abstract
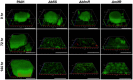
Biofilm formation represents a critical strategy whereby bacteria can tolerate otherwise damaging environmental stressors and antimicrobial insults. While the mechanisms bacteria use to establish a biofilm and disperse from these communities have been well-studied, we have only a limited understanding of the mechanisms required to maintain these multicellular communities. Indeed, until relatively recently, it was not clear that maintaining a mature biofilm could be considered an active, regulated process with dedicated machinery. Using Pseudomonas aeruginosa as a model system, we review evidence from recent studies that support the model that maintenance of these persistent, surface-attached communities is indeed an active process. Biofilm maintenance mechanisms include transcriptional regulation and second messenger signaling (including the production of extracellular polymeric substances). We also discuss energy-conserving pathways that play a key role in the maintenance of these communities. We hope to highlight the need for further investigation to uncover novel biofilm maintenance pathways and suggest the possibility that such pathways can serve as novel antibiofilm targets.
Keywords: Pseudomonas aeruginosa; biofilm; c-di-GMP; maintenance; metabolism; peroxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
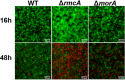
Pseudomonas aeruginosa Uses c-di-GMP Phosphodiesterases RmcA and MorA To Regulate Biofilm Maintenance.mBio. 2021 Feb 2;12(1):e03384-20. doi: 10.1128/mBio.03384-20. mBio. 2021. PMID: 33531388 Free PMC article.
-
The Diguanylate Cyclase YfiN of Pseudomonas aeruginosa Regulates Biofilm Maintenance in Response to Peroxide.J Bacteriol. 2022 Jan 18;204(1):e0039621. doi: 10.1128/JB.00396-21. Epub 2021 Oct 25. J Bacteriol. 2022. PMID: 34694901 Free PMC article.
-
Heterogeneity in surface sensing suggests a division of labor in Pseudomonas aeruginosa populations.Elife. 2019 Jun 10;8:e45084. doi: 10.7554/eLife.45084. Elife. 2019. PMID: 31180327 Free PMC article.
-
The Pseudomonas aeruginosa Biofilm Matrix Protein CdrA Has Similarities to Other Fibrillar Adhesin Proteins.J Bacteriol. 2023 May 25;205(5):e0001923. doi: 10.1128/jb.00019-23. Epub 2023 Apr 26. J Bacteriol. 2023. PMID: 37098957 Free PMC article. Review.
-
Metabolomic profiling of bacterial biofilm: trends, challenges, and an emerging antibiofilm target.World J Microbiol Biotechnol. 2023 May 31;39(8):212. doi: 10.1007/s11274-023-03651-y. World J Microbiol Biotechnol. 2023. PMID: 37256458 Review.
Cited by
-
Editorial: Role of microbial biofilm in infections.Front Cell Infect Microbiol. 2023 Jun 19;13:1231607. doi: 10.3389/fcimb.2023.1231607. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37404726 Free PMC article. No abstract available.
-
GepA, a GGDEF-EAL protein, regulates biofilm formation and swimming motility in Vibrio parahaemolyticus.Arch Microbiol. 2025 Mar 22;207(5):99. doi: 10.1007/s00203-025-04282-7. Arch Microbiol. 2025. PMID: 40119885
-
H-NOX Influences Biofilm Formation, Central Metabolism, and Quorum Sensing in Paracoccus denitrificans.J Proteome Res. 2024 Nov 1;23(11):4988-5000. doi: 10.1021/acs.jproteome.4c00466. Epub 2024 Oct 6. J Proteome Res. 2024. PMID: 39370609 Free PMC article.
-
It's time to write a minireview.J Bacteriol. 2024 May 23;206(5):e0011324. doi: 10.1128/jb.00113-24. Epub 2024 Apr 16. J Bacteriol. 2024. PMID: 38624220 Free PMC article. No abstract available.
-
Multispecies biofilm architecture determines bacterial exposure to phages.PLoS Biol. 2022 Dec 22;20(12):e3001913. doi: 10.1371/journal.pbio.3001913. eCollection 2022 Dec. PLoS Biol. 2022. PMID: 36548227 Free PMC article.
References
-
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. 10.1126/science.1211037. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

