Comparative Genomics of Cyclic di-GMP Metabolism and Chemosensory Pathways in Shewanella algae Strains: Novel Bacterial Sensory Domains and Functional Insights into Lifestyle Regulation
- PMID: 35311563
- PMCID: PMC9040814
- DOI: 10.1128/msystems.01518-21
Comparative Genomics of Cyclic di-GMP Metabolism and Chemosensory Pathways in Shewanella algae Strains: Novel Bacterial Sensory Domains and Functional Insights into Lifestyle Regulation
Abstract
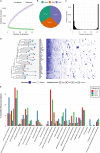
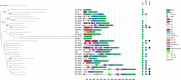
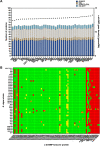
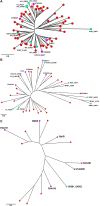
Shewanella spp. play important ecological and biogeochemical roles, due in part to their versatile metabolism and swift integration of stimuli. While Shewanella spp. are primarily considered environmental microbes, Shewanella algae is increasingly recognized as an occasional human pathogen. S. algae shares the broad metabolic and respiratory repertoire of Shewanella spp. and thrives in similar ecological niches. In S. algae, nitrate and dimethyl sulfoxide (DMSO) respiration promote biofilm formation strain specifically, with potential implication of taxis and cyclic diguanosine monophosphate (c-di-GMP) signaling. Signal transduction systems in S. algae have not been investigated. To fill these knowledge gaps, we provide here an inventory of the c-di-GMP turnover proteome and chemosensory networks of the type strain S. algae CECT 5071 and compare them with those of 41 whole-genome-sequenced clinical and environmental S. algae isolates. Besides comparative analysis of genetic content and identification of laterally transferred genes, the occurrence and topology of c-di-GMP turnover proteins and chemoreceptors were analyzed. We found S. algae strains to encode 61 to 67 c-di-GMP turnover proteins and 28 to 31 chemoreceptors, placing S. algae near the top in terms of these signaling capacities per Mbp of genome. Most c-di-GMP turnover proteins were predicted to be catalytically active; we describe in them six novel N-terminal sensory domains that appear to control their catalytic activity. Overall, our work defines the c-di-GMP and chemosensory signal transduction pathways in S. algae, contributing to a better understanding of its ecophysiology and establishing S. algae as an auspicious model for the analysis of metabolic and signaling pathways within the genus Shewanella. IMPORTANCE Shewanella spp. are widespread aquatic bacteria that include the well-studied freshwater model strain Shewanella oneidensis MR-1. In contrast, the physiology of the marine and occasionally pathogenic species Shewanella algae is poorly understood. Chemosensory and c-di-GMP signal transduction systems integrate environmental stimuli to modulate gene expression, including the switch from a planktonic to sessile lifestyle and pathogenicity. Here, we systematically dissect the c-di-GMP proteome and chemosensory pathways of the type strain S. algae CECT 5071 and 41 additional S. algae isolates. We provide insights into the activity and function of these proteins, including a description of six novel sensory domains. Our work will enable future analyses of the complex, intertwined c-di-GMP metabolism and chemotaxis networks of S. algae and their ecophysiological role.
Keywords: Shewanella; c-di-GMP; chemotaxis; sensing; signal transduction; whole-genome sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Reduction of alternative electron acceptors drives biofilm formation in Shewanella algae.NPJ Biofilms Microbiomes. 2021 Jan 27;7(1):9. doi: 10.1038/s41522-020-00177-1. NPJ Biofilms Microbiomes. 2021. PMID: 33504806 Free PMC article.
-
Identification of a Diguanylate Cyclase That Facilitates Biofilm Formation on Electrodes by Shewanella oneidensis MR-1.Appl Environ Microbiol. 2021 Apr 13;87(9):e00201-21. doi: 10.1128/AEM.00201-21. Print 2021 Apr 13. Appl Environ Microbiol. 2021. PMID: 33637573 Free PMC article.
-
Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi.Biochemistry. 2012 Mar 13;51(10):2087-99. doi: 10.1021/bi201753f. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22360279
-
Regulation of c-di-GMP metabolism in biofilms.Future Microbiol. 2009 Apr;4(3):341-58. doi: 10.2217/fmb.09.7. Future Microbiol. 2009. PMID: 19327118 Review.
-
Tale of two metal reducers: comparative proteome analysis of Geobacter sulferreducens PCA and Shewanella oneidensis MR-1.Methods Biochem Anal. 2006;49:97-111. Methods Biochem Anal. 2006. PMID: 16929676 Review.
Cited by
-
Bacterial sensor evolved by decreasing complexity.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2409881122. doi: 10.1073/pnas.2409881122. Epub 2025 Jan 29. Proc Natl Acad Sci U S A. 2025. PMID: 39879239 Free PMC article.
-
Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina.Int J Mol Sci. 2024 Aug 22;25(16):9111. doi: 10.3390/ijms25169111. Int J Mol Sci. 2024. PMID: 39201797 Free PMC article.
-
Gas and light: triggers of c-di-GMP-mediated regulation.FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad034. doi: 10.1093/femsre/fuad034. FEMS Microbiol Rev. 2023. PMID: 37339911 Free PMC article. Review.
-
Structural and functional diversity of sensor domains in bacterial transmembrane receptors.Trends Microbiol. 2025 Jul;33(7):796-809. doi: 10.1016/j.tim.2025.02.019. Epub 2025 Mar 21. Trends Microbiol. 2025. PMID: 40121131 Review.
-
Phylogenetic Analysis and Characterization of Diguanylate Cyclase and Phosphodiesterase in Planktonic Filamentous Cyanobacterium Arthrospira sp.Int J Mol Sci. 2023 Oct 16;24(20):15210. doi: 10.3390/ijms242015210. Int J Mol Sci. 2023. PMID: 37894891 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

