Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies
- PMID: 35311573
- PMCID: PMC9045172
- DOI: 10.1128/spectrum.01496-21
Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies
Erratum in
-
Erratum for Li et al., "Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies".Microbiol Spectr. 2022 Aug 31;10(4):e0228222. doi: 10.1128/spectrum.02282-22. Epub 2022 Jul 5. Microbiol Spectr. 2022. PMID: 35862987 Free PMC article. No abstract available.
Abstract
In recent years, the chikungunya virus (CHIKV) has continued to spread from local epidemics to nonnative habitats until eventually reaching pandemic status. Nonendemic areas such as China have also emerged as potential epidemic areas of CHIKV. Serological detection of CHIKV is the key to diagnosing and controlling the prevalence of this virus. In this study, we review the progress of the serological detection of the envelope (E) protein in CHIKV, and we provide a novel research assay and ideas for the serological detection of CHIKV. The luciferase immunosorbent assay (LISA) does not require species-specific labeled secondary antibodies for detection, making it universally suitable for tracking samples from various animals or carriers. At present, most research on CHIKV antigen detection technology tends to combine two or more proteins to avoid the decrease in detection ability caused by antigen mutation. Our results indicate that two or more kinds of CHIKV E antigens combined with LISA detection can improve the detection rate of anti-CHIKV immunoglobulin G (IgG) antibodies in CHIKV-infected patient sera and detect antibodies in the early stage of infection accurately and sensitively. After 235 days of infection, the anti-CHIKV IgG antibodies could still be detected in CHIKV-infected patients. All serum samples were tested with a detection rate of 100% after combining various recombinant CHIKV E antigens. Our proposed CHIKV-specific LISA could be a useful tool for serum diagnosis of CHIKV infection and serum epidemic research in areas where CHIKV is endemic, which would help to manage potential epidemics in the future. IMPORTANCE At present, chikungunya virus (CHIKV) is still circulating in some parts of the world, and mutated strains have emerged, making it easier for the virus to spread among humans. With the continuous variation of CHIKV, its antigen variation leads to the decline of detection ability. In addition, the risk of transmission of CHIKV in areas where CHIKV is not endemic, such as China, has increased dramatically, which compels us to enhance the detection capacity of CHIKV and continuously monitor CHIKV antibody levels in the population. Real-time quantitative PCR (RT-PCR) detection technology will not be reliable when the infection time is chronic or in subclinical infection due to decreases in virus concentration, and an antibody detection technology must be adopted. In this study, multiple CHIKV envelope (E) antigens were used to detect anti-CHIKV IgG antibodies in serum for the first time. The new assay is characterized by convenient operation, high detection rate, and high sensitivity and has significance for early warning and monitoring. Moreover, it contributes to the prevention and control of CHIKV.
Keywords: CHIKV antibodies; chikungunya virus (CHIKV); envelope (E) antigens; envelope protein; luciferase immunosorbent assay (LISA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

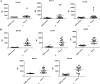
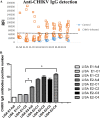
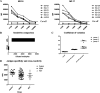

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

