Comparison of Saliva and Midturbinate Swabs for Detection of SARS-CoV-2
- PMID: 35311575
- PMCID: PMC9045394
- DOI: 10.1128/spectrum.00128-22
Comparison of Saliva and Midturbinate Swabs for Detection of SARS-CoV-2
Abstract
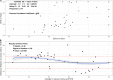
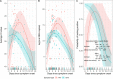
Saliva is an attractive sample for detecting SARS-CoV-2. However, contradictory reports exist concerning the sensitivity of saliva versus nasal swabs. We followed close contacts of COVID-19 cases for up to 14 days from the last exposure and collected self-reported symptoms, midturbinate swabs (MTS), and saliva every 2 or 3 days. Ct values, viral load, and frequency of viral detection by MTS and saliva were compared. Fifty-eight contacts provided 200 saliva-MTS pairs, and 14 contacts (13 with symptoms) had one or more positive samples. Saliva and MTS had similar rates of viral detection (P = 0.78) and substantial agreement (κ = 0.83). However, sensitivity varied significantly with time since symptom onset. Early on (days -3 to 2), saliva had 12 times (95% CI: 1.2, 130) greater likelihood of viral detection and 3.2 times (95% CI: 2.8, 3.8) higher RNA copy numbers compared to MTS. After day 2 of symptoms, there was a nonsignificant trend toward greater sensitivity using MTS. Saliva and MTS demonstrated high agreement making saliva a suitable alternative to MTS for SARS-CoV-2 detection. Saliva was more sensitive early in the infection when the transmission was most likely to occur, suggesting that it may be a superior and cost-effective screening tool for COVID-19. IMPORTANCE The findings of this manuscript are increasingly important with new variants that appear to have shorter incubation periods emerging, which may be more prone to detection in saliva before detection in nasal swabs. Therefore, there is an urgent need to provide the science to support the use of a detection method that is highly sensitive and widely acceptable to the public to improve screening rates and early detection. The manuscript presents the first evidence that saliva-based RT-PCR is more sensitive than MTS-based RT-PCR in detecting SARS-CoV-2 during the presymptomatic period - the critical period for unwitting onward transmission. Considering other advantages of saliva samples, including the lower cost, greater acceptability within the general population, and less risk to health care workers, our findings further supported the use of saliva to identify presymptomatic infection and prevent transmission of the virus.
Keywords: COVID-19; SARS-CoV-2; midturbinate swab; polymerase chain reaction; saliva.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Detection and Stability of SARS-CoV-2 in Three Self-Collected Specimen Types: Flocked Midturbinate Swab (MTS) in Viral Transport Media, Foam MTS, and Saliva.Microbiol Spectr. 2022 Jun 29;10(3):e0103322. doi: 10.1128/spectrum.01033-22. Epub 2022 Jun 6. Microbiol Spectr. 2022. PMID: 35665629 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Saliva is Comparable to Nasopharyngeal Swabs for Molecular Detection of SARS-CoV-2.Microbiol Spectr. 2021 Sep 3;9(1):e0016221. doi: 10.1128/Spectrum.00162-21. Epub 2021 Aug 18. Microbiol Spectr. 2021. PMID: 34406838 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs : A Systematic Review and Meta-analysis.Ann Intern Med. 2021 Apr;174(4):501-510. doi: 10.7326/M20-6569. Epub 2021 Jan 12. Ann Intern Med. 2021. PMID: 33428446 Free PMC article.
Cited by
-
A multiplexed, paired-pooled droplet digital PCR assay for detection of SARS-CoV-2 in saliva.Sci Rep. 2023 Feb 22;13(1):3075. doi: 10.1038/s41598-023-29858-5. Sci Rep. 2023. PMID: 36813822 Free PMC article.
-
Saliva as alternative to naso-oropharyngeal swab for SARS-CoV-2 detection by RT-qPCR: a multicenter cross-sectional diagnostic validation study.Sci Rep. 2022 Jul 23;12(1):12612. doi: 10.1038/s41598-022-16849-1. Sci Rep. 2022. PMID: 35871257 Free PMC article.
-
Molecular diagnostic approaches for SARS-CoV-2 detection and pathophysiological consequences.Mol Biol Rep. 2023 Dec;50(12):10367-10382. doi: 10.1007/s11033-023-08844-0. Epub 2023 Oct 10. Mol Biol Rep. 2023. PMID: 37817022 Review.
-
Daily SARS-CoV-2 Nasal Antigen Tests Miss Infected and Presumably Infectious People Due to Viral Load Differences among Specimen Types.Microbiol Spectr. 2023 Aug 17;11(4):e0129523. doi: 10.1128/spectrum.01295-23. Epub 2023 Jun 14. Microbiol Spectr. 2023. PMID: 37314333 Free PMC article.
-
Antigen concentration, viral load, and test performance for SARS-CoV-2 in multiple specimen types.PLoS One. 2023 Jul 19;18(7):e0287814. doi: 10.1371/journal.pone.0287814. eCollection 2023. PLoS One. 2023. PMID: 37467188 Free PMC article.
References
-
- Centers for Disease Control and Prevention. 2021. Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. Centers for Disease Control and Prevention, Atlanta, Georgia, USA. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim....
-
- Jamal AJ, Mozafarihashjin M, Coomes E, Anceva-Sami S, Barati S, Crowl G, Faheem A, Farooqi L, Kandel CE, Khan S, Li AX, Mistry H, Paterson A, Plenderleith S, Prost K, Poutanen S, Powis J, Schryer R, Taylor M, Yip L, Zhong XZ, McGeer AJ, Mubareka S, Toronto Invasive Bacterial Diseases Network COVID-19 Investigators . 2021. Sensitivity of midturbinate versus nasopharyngeal swabs for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol 42:1001–1003. doi:10.1017/ice.2020.1326. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

