Surveying the Epigenetic Landscape of Tuberculosis in Alveolar Macrophages
- PMID: 35311579
- PMCID: PMC9119088
- DOI: 10.1128/iai.00522-21
Surveying the Epigenetic Landscape of Tuberculosis in Alveolar Macrophages
Abstract
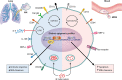
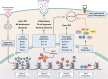
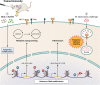
Tuberculosis (TB) remains the leading cause of bacterial disease-related death and is among the top 10 overall causes of death worldwide. The complex nature of this infectious lung disease has proven difficult to treat, and significant research efforts are now evaluating the feasibility of host-directed, adjunctive therapies. An attractive approach in host-directed therapy targets host epigenetics, or gene regulation, to redirect the immune response in a host-beneficial manner. Substantial evidence exists demonstrating that host epigenetics are dysregulated during TB and that epigenetic-based therapies may be highly effective to treat TB. However, the caveat is that much of the knowledge that exists on the modulation of the host epigenome during TB has been gained using in vitro, small-animal, or blood-derived cell models, which do not accurately reflect the pulmonary nature of the disease. In humans, the first and major target cells of Mycobacterium tuberculosis are alveolar macrophages (AM). As such, their response to infection and treatment is clinically relevant and ultimately drives the outcome of disease. In this review, we compare the fundamental differences between AM and circulating monocyte-derived macrophages in the context of TB and summarize the recent advances in elucidating the epigenomes of these cells, including changes to the transcriptome, DNA methylome, and chromatin architecture. We will also discuss trained immunity in AM as a new and emerging field in TB research and provide some perspectives for the translational potential of targeting host epigenetics as an alternative TB therapy.
Keywords: DNA methylation; Mycobacterium; alveolar macrophages; epigenetics; histone modifications; host-directed therapy; lung immune cells; trained immunity; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO. 2021. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland.
-
- Chaisson R. 2020. Statin adjunctive therapy for TB (StAT-TB). https://clinicaltrials.gov/ct2/show/NCT02968927?term=hostdirected+therap....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

