Oral delivery of the intracellular domain of the insulinoma-associated protein 2 (IA-2ic) by bacterium-like particles (BLPs) prevents type 1 diabetes mellitus in NOD mice
- PMID: 35311607
- PMCID: PMC8942491
- DOI: 10.1080/10717544.2022.2053760
Oral delivery of the intracellular domain of the insulinoma-associated protein 2 (IA-2ic) by bacterium-like particles (BLPs) prevents type 1 diabetes mellitus in NOD mice
Abstract
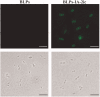
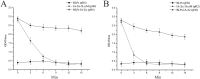
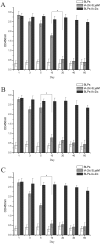
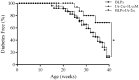
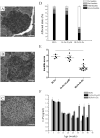
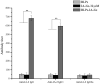
Antigen-specific immune tolerance, which possesses great potential in preventing or curing type 1 diabetes mellitus (T1DM), can be induced by oral vaccination with T1DM-related autoantigens. However, direct administration of autoantigens via oral route exhibits a low tolerance-inducing effect as a result of the digestion of protein antigens in the gastrointestinal tract (GIT) and therefore, a large dosage of autoantigens may be needed. In this study, bacterium-like particles (BLPs) made from food-grade lactic acid bacteria were used to deliver the intracellular domain of the insulinoma-associated protein 2 (IA-2ic). For this purpose, BLPs-IA-2ic vaccine in which IA-2ic bound to the surface of BLPs was constructed. BLPs enhanced the stability of the delivered IA-2ic based on the stability analysis in vitro. Oral administration of BLPs-IA-2ic significantly reduced T1DM incidence in NOD mice. The mice fed BLPs-IA-2ic exhibited a significant reduction in insulitis and preserved the ability to secrete insulin. Immunologic analysis showed that oral vaccination with BLPs-IA-2ic induced antigen-specific T cell tolerance. The results revealed that the successful induction of immune tolerance was dependent on the immune deviation (in favor of T helper 2 responses) and CD4+CD25+FoxP3+ regulatory T cells. Hence, oral vaccination with BLPs-IA-2ic shows potential for application in preventing T1DM.
Keywords: Type 1 diabetes mellitus; antigen delivery; bacterium-like particles; insulinoma-associated protein 2; oral tolerance.
Conflict of interest statement
The authors report no conflict of interest.
Figures



Similar articles
-
Oral delivery of bi-autoantigens by bacterium-like particles (BLPs) against autoimmune diabetes in NOD mice.Drug Deliv. 2023 Dec;30(1):2173339. doi: 10.1080/10717544.2023.2173339. Drug Deliv. 2023. PMID: 36719009 Free PMC article.
-
Bacterium-like particles derived from probiotics: progress, challenges and prospects.Front Immunol. 2023 Oct 6;14:1263586. doi: 10.3389/fimmu.2023.1263586. eCollection 2023. Front Immunol. 2023. PMID: 37868963 Free PMC article. Review.
-
Oral delivery of single-chain insulin (SCI-59) analog by bacterium-like particles (BLPs) induces oral tolerance and prevents autoimmune diabetes in NOD mice.Immunol Lett. 2019 Oct;214:37-44. doi: 10.1016/j.imlet.2019.08.008. Epub 2019 Aug 29. Immunol Lett. 2019. PMID: 31473255
-
Engineering and expression of the intracellular domain of insulinoma-associated tyrosine phosphatase (IA-2ic), a type 1 diabetes autoantigen, in plants.Transgenic Res. 2007 Feb;16(1):77-84. doi: 10.1007/s11248-006-9033-3. Epub 2006 Nov 11. Transgenic Res. 2007. PMID: 17103024
-
International Workshop on Lessons From Animal Models for Human Type 1 Diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice.Diabetes. 2001 Nov;50(11):2451-8. doi: 10.2337/diabetes.50.11.2451. Diabetes. 2001. PMID: 11679421 Review.
Cited by
-
Vaccines for immune tolerance against autoimmune disease.Adv Drug Deliv Rev. 2023 Dec;203:115140. doi: 10.1016/j.addr.2023.115140. Epub 2023 Nov 18. Adv Drug Deliv Rev. 2023. PMID: 37980949 Free PMC article. Review.
-
Oral delivery of bi-autoantigens by bacterium-like particles (BLPs) against autoimmune diabetes in NOD mice.Drug Deliv. 2023 Dec;30(1):2173339. doi: 10.1080/10717544.2023.2173339. Drug Deliv. 2023. PMID: 36719009 Free PMC article.
-
Bacterium-like particles derived from probiotics: progress, challenges and prospects.Front Immunol. 2023 Oct 6;14:1263586. doi: 10.3389/fimmu.2023.1263586. eCollection 2023. Front Immunol. 2023. PMID: 37868963 Free PMC article. Review.
-
Challenges and opportunities in delivering oral peptides and proteins.Expert Opin Drug Deliv. 2023 Jul-Dec;20(10):1349-1369. doi: 10.1080/17425247.2023.2237408. Epub 2023 Jul 17. Expert Opin Drug Deliv. 2023. PMID: 37450427 Free PMC article. Review.
-
Advanced Delivery Strategies for Immunotherapy in Type I Diabetes Mellitus.BioDrugs. 2023 May;37(3):331-352. doi: 10.1007/s40259-023-00594-6. Epub 2023 May 13. BioDrugs. 2023. PMID: 37178431 Free PMC article. Review.
References
-
- Bilate AM, Lafaille JJ. (2012). Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30:733–58. - PubMed
-
- Bonifacio E, Atkinson M, Eisenbarth G, et al. (2001). International workshop on lessons from animal models for human type 1 diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice. Diabetes 50:2451–8. - PubMed
-
- Bonifacio E, Ziegler AG, Klingensmith G, et al. (2015). Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA 313:1541–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
