Conflicts of interest for members of the US 2020 dietary guidelines advisory committee
- PMID: 35311630
- PMCID: PMC10966930
- DOI: 10.1017/S1368980022000672
Conflicts of interest for members of the US 2020 dietary guidelines advisory committee
Abstract
Objectives: To measure incidence of conflicts of interest (COI) with food and pharmaceutical industry actors on the advisory committee for the 2020-2025 US Dietary Guidelines for Americans (DGA) and assess the adequacy of current mechanisms to disclose and manage COI among the committee's members.
Design: We compiled longitudinal data from archival sources on connections between members of the DGA's advisory committee and actors. We hypothesised that these committee members, who oversee the science for the most influential dietary policy in the USA, might have significant COI that would be relevant to their decision making. Disclosure of COI on this committee was recommended in 2017 by the National Academies of Sciences in order to increase transparency and manage bias, but public disclosure of the committee's COI does not appear to have taken place.
Setting: The committee was composed of twenty experts.
Participants: None.
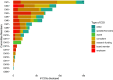
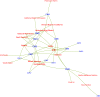
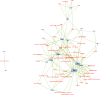
Results: Our analysis found that 95 % of the committee members had COI with the food and/or pharmaceutical industries and that particular actors, including Kellogg, Abbott, Kraft, Mead Johnson, General Mills, Dannon and the International Life Sciences, had connections with multiple members. Research funding and membership of an advisory/executive board jointly accounted for more than 60 % of the total number of COI documented.
Conclusions: Trustworthy dietary guidelines result from a transparent, objective and science-based, process. Our analysis has shown that the significant and widespread COI on the committee prevent the DGA from achieving the recommended standard for transparency without mechanisms in place to make this information publicly available.
Keywords: Commercial determinants of health; Conflicts of interest; Dietary guidelines.
Conflict of interest statement
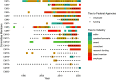
For this study, we defined COI as relationships between a DGAC member and an industry actor in a given year. We documented the year in which the COI was disclosed as the year for which the COI existed, even if the relationship between the DGAC members and the organisation might have been maintained for a longer period of time than that disclosed. For example, a 5-year research grant yielding one published paper was only considered once as a COI (for the publication of the article), given that we lacked evidence for the whole 5-year period. This drawback is only avoidable for those cases where the duration of the COI was disclosed (e.g. start and end date of the grant). Furthermore, lacking evidence to the contrary, we considered funding from industry to be a COI for any DGAC member who is a co-author on a study sponsored by industry. And on the contrary, if the relationship or grant was mentioned in multiple publications in the same year, we counted it once. This approach does not distinguish between cases where a DGAC member might have received more than one grant in the same year from the same industry actor, as we count that as a single instance of a COI.
We argue that the time dimension is important in order to shed light on long-term relationships between industry and DGAC members. Therefore, we considered COI without date restrictions, allowing us to go as far back in time as information is publicly available.
We took a conservative approach using exclusively primary data to obtain evidence of a COI. We considered primary data sources as those platforms where information about COI is disclosed either directly by a DGAC member (e.g. scientific publication or a Curriculum Vitae) or by the institutions to which they were affiliated (e.g. bios on institutional websites). Primary data sources were excluded where a COI was discussed without a reference to the original information source.
We focused on the COI of DGAC members with corporate actors from the food, drink, and pharmaceutical industries, as well as third parties working with them such as trade associations or front groups. We included pharmaceutical companies because some sell infant nutrition products and often offer devices or drugs that compete with food-based solutions to chronic diseases. We searched for information specifically on the DGAC members, not their families or other third parties, as included in Form 450.
Below, we expand on the iterative process through which COI were identified, collected and documented.
There are no conflicts of interest.
Figures


References
-
- USDA/HHS (2020) Who’s Involved – Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/about-dietary-guidelines/process (accessed June 2021).
-
- USDA/HHS (2020) Purpose – Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/about-dietary-guidelines/purpose-dieta... (accessed June 2021).
-
- United States 101st Congress (1990) National Nutrition Monitoring and Related Research Act of 1990 – Section 301 of PL 101–445 (7 USC 5341). https://www.congress.gov/bill/101st-congress/house-bill/160 (accessed June 2021).
-
- U.S. Department of Agriculture (2021) USDA Budget Explanatory Notes: Food and Nutrition Service. https://www.usda.gov/sites/default/files/documents/32fns2021notes.pdf (accessed June 2021).
-
- USDA/HHS (2020) History of the Dietary Guidelines – Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/about-dietary-guidelines/history-dieta... (accessed June 2021).
LinkOut - more resources
Full Text Sources

