Inhibiting USP16 rescues stem cell aging and memory in an Alzheimer's model
- PMID: 35311644
- PMCID: PMC9122497
- DOI: 10.7554/eLife.66037
Inhibiting USP16 rescues stem cell aging and memory in an Alzheimer's model
Abstract
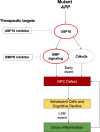
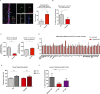
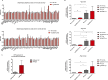
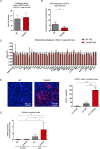
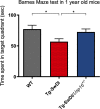
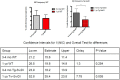
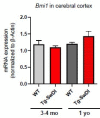
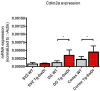
Alzheimer's disease (AD) is a progressive neurodegenerative disease observed with aging that represents the most common form of dementia. To date, therapies targeting end-stage disease plaques, tangles, or inflammation have limited efficacy. Therefore, we set out to identify a potential earlier targetable phenotype. Utilizing a mouse model of AD and human fetal cells harboring mutant amyloid precursor protein, we show cell intrinsic neural precursor cell (NPC) dysfunction precedes widespread inflammation and amyloid plaque pathology, making it the earliest defect in the evolution of the disease. We demonstrate that reversing impaired NPC self-renewal via genetic reduction of USP16, a histone modifier and critical physiological antagonist of the Polycomb Repressor Complex 1, can prevent downstream cognitive defects and decrease astrogliosis in vivo. Reduction of USP16 led to decreased expression of senescence gene Cdkn2a and mitigated aberrant regulation of the Bone Morphogenetic Signaling (BMP) pathway, a previously unknown function of USP16. Thus, we reveal USP16 as a novel target in an AD model that can both ameliorate the NPC defect and rescue memory and learning through its regulation of both Cdkn2a and BMP signaling.
Keywords: Alzheimer's; mouse; neural stem cells; neurodegeneration; regenerative medicine; stem cells.
© 2022, Reinitz et al.
Conflict of interest statement
FR, EC, JA, RJ, MC filer of a provisional patent: U.S.Provisional Application No. 63/124,644 titled "Modulating BMP signaling in the treatment of Alzheimer's disease", BN, MA is the co-founder of Dorian Therapeutics. Dorian therapeutics was incorporated in June 2018 and it is an early stage anti-aging company that focuses on the process of cellular senescence. Most of the experiments were performed before the company was formed, BC, NG, KL, WH, SK, DQ, KP, AA, MM, HH, SQ No competing interests declared
Figures
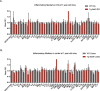
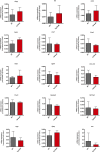
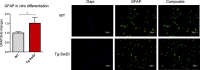

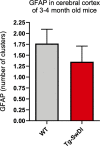
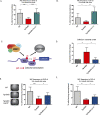
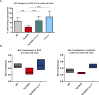



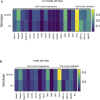
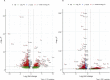



Similar articles
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Neuronal Protein Tyrosine Phosphatase 1B Hastens Amyloid β-Associated Alzheimer's Disease in Mice.J Neurosci. 2020 Feb 12;40(7):1581-1593. doi: 10.1523/JNEUROSCI.2120-19.2019. Epub 2020 Jan 8. J Neurosci. 2020. PMID: 31915254 Free PMC article.
-
Usp16 contributes to somatic stem-cell defects in Down's syndrome.Nature. 2013 Sep 19;501(7467):380-4. doi: 10.1038/nature12530. Epub 2013 Sep 11. Nature. 2013. PMID: 24025767 Free PMC article.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Behavioral and Neuronal Characterizations, across Ages, of the TgSwDI Mouse Model of Alzheimer's Disease.Genes (Basel). 2023 Dec 28;15(1):47. doi: 10.3390/genes15010047. Genes (Basel). 2023. PMID: 38254938 Free PMC article.
-
Structural and mechanistic basis for nucleosomal H2AK119 deubiquitination by single-subunit deubiquitinase USP16.Nat Struct Mol Biol. 2024 Nov;31(11):1745-1755. doi: 10.1038/s41594-024-01342-2. Epub 2024 Jun 25. Nat Struct Mol Biol. 2024. PMID: 38918638
-
Small Molecule Screen Identifies Non-catalytic USP3 Chemical Handle.ACS Omega. 2023 Dec 15;9(1):917-924. doi: 10.1021/acsomega.3c07070. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38222562 Free PMC article.
-
Auto-branch multi-task learning for simultaneous prediction of multiple correlated traits associated with Alzheimer's disease.Front Genet. 2025 Jun 10;16:1538544. doi: 10.3389/fgene.2025.1538544. eCollection 2025. Front Genet. 2025. PMID: 40557283 Free PMC article.
-
The Pleiotropic Ubiquitin-Specific Peptidase 16 and Its Many Substrates.Cells. 2023 Mar 13;12(6):886. doi: 10.3390/cells12060886. Cells. 2023. PMID: 36980227 Free PMC article. Review.
References
-
- Adorno M, Sikandar S, Mitra SS, Kuo A, Nicolis Di Robilant B, Haro-Acosta V, Ouadah Y, Quarta M, Rodriguez J, Qian D, Reddy VM, Cheshier S, Garner CC, Clarke MF. Usp16 contributes to somatic stem-cell defects in Down’s syndrome. Nature. 2013;501:380–384. doi: 10.1038/nature12530. - DOI - PMC - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

