Rapid Point-of-Care Genotyping to Avoid Aminoglycoside-Induced Ototoxicity in Neonatal Intensive Care
- PMID: 35311942
- PMCID: PMC8938898
- DOI: 10.1001/jamapediatrics.2022.0187
Rapid Point-of-Care Genotyping to Avoid Aminoglycoside-Induced Ototoxicity in Neonatal Intensive Care
Abstract
Importance: Aminoglycosides are commonly prescribed antibiotics used for the treatment of neonatal sepsis. The MT-RNR1 m.1555A>G variant predisposes to profound aminoglycoside-induced ototoxicity (AIO). Current genotyping approaches take several days, which is unfeasible in acute settings.
Objective: To develop a rapid point-of-care test (POCT) for the m.1555A>G variant before implementation of this technology in the acute neonatal setting to guide antibiotic prescribing and avoid AIO.
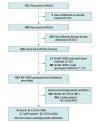
Design, setting, and participants: This pragmatic prospective implementation trial recruited neonates admitted to 2 large neonatal intensive care units between January 6, 2020, and November 30, 2020, in the UK.
Interventions: Neonates were tested for the m.1555A>G variant via the rapid POCT on admission to the neonatal intensive care unit.
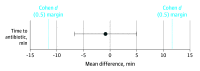
Main outcomes and measures: The primary outcome assessed the proportion of neonates successfully tested for the variant of all infants prescribed antibiotics. Secondary outcomes measured whether implementation was negatively associated with routine clinical practice and the performance of the system. The study was statistically powered to detect a significant difference between time to antibiotic administration before and after implementation of the MT-RNR1 POCT.
Results: A total of 751 neonates were recruited and had a median (range) age of 2.5 (0-198) days. The MT-RNR1 POCT was able to genotype the m.1555A>G variant in 26 minutes. Preclinical validation demonstrated a 100% sensitivity (95% CI, 93.9%-100.0%) and specificity (95% CI, 98.5%-100.0%). Three participants with the m.1555A>G variant were identified, all of whom avoided aminoglycoside antibiotics. Overall, 424 infants (80.6%) receiving antibiotics were successfully tested for the variant, and the mean time to antibiotics was equivalent to previous practice.
Conclusions and relevance: The MT-RNR1 POCT was integrated without disrupting normal clinical practice, and genotype was used to guide antibiotic prescription and avoid AIO. This approach identified the m.1555A>G variant in a practice-changing time frame, and wide adoption could significantly reduce the burden of AIO.
Conflict of interest statement
Figures
Comment in
-
Genetic Testing in Newborns Moves From Rare to Routine Application.JAMA Pediatr. 2022 May 1;176(5):448-449. doi: 10.1001/jamapediatrics.2022.0184. JAMA Pediatr. 2022. PMID: 35311940 No abstract available.
-
Options for Detecting Risk of Aminoglycoside-Induced Ototoxicity in Neonates-Reply.JAMA Pediatr. 2022 Aug 1;176(8):827-828. doi: 10.1001/jamapediatrics.2022.2062. JAMA Pediatr. 2022. PMID: 35727572 No abstract available.
-
Options for Detecting Risk of Aminoglycoside-Induced Ototoxicity in Neonates.JAMA Pediatr. 2022 Aug 1;176(8):828. doi: 10.1001/jamapediatrics.2022.2065. JAMA Pediatr. 2022. PMID: 35727587 No abstract available.

