Reporting of Participant Race and Ethnicity in Published US Pediatric Clinical Trials From 2011 to 2020
- PMID: 35311946
- PMCID: PMC8938892
- DOI: 10.1001/jamapediatrics.2022.0142
Reporting of Participant Race and Ethnicity in Published US Pediatric Clinical Trials From 2011 to 2020
Abstract
Importance: Equitable representation of participants who are members of racial and ethnic minority groups in clinical trials enhances inclusivity in the scientific process and generalizability of results.
Objective: To assess participant race and ethnicity in pediatric clinical trials published from 2011 to 2020.
Design, setting, and participants: This cross-sectional study examined articles reporting pediatric clinical trials conducted in the US published in 5 leading general pediatric and 5 leading general medical journals from January 1, 2011, to December 31, 2020.
Main outcomes and measures: Reporting of participant race and ethnicity and comparison of enrolled participants vs US census populations of pediatric racial and ethnic groups in published clinical trials.
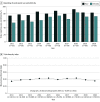
Results: The study included 612 articles reporting pediatric clinical trials during the study period, with 565 618 total participants (median per trial, 200 participants [IQR, 90-571 participants]). Of the 612 articles, 486 (79.4%) reported participant race and 338 (55.2%) reported participant ethnicity. From 2011 to 2020, relative rates of reporting of participant race increased by 7.9% per year (95% CI, 0.2%-16.3% per year) and reporting of ethnicity increased by 11.4% per year (95% CI, 4.8%-18.4% per year). Among articles reporting race and ethnicity, the method of assignment was not reported in 261 of 511 articles (51.1%) and 207 of 359 articles (57.7%), respectively. Black/African American children were enrolled proportionally more than the US population of Black/African American children (odds ratio [OR], 1.88; 95% CI, 1.87-1.89). Hispanic/Latino children were enrolled commensurately with the US population of Hispanic/Latino children (OR, 1.02; 95% CI, 1.01-1.03). American Indian/Alaska Native (OR, 0.82; 95% CI, 0.79-0.85), Asian (OR, 0.56; 95% CI, 0.55-0.57), and Native Hawaiian/Pacific Islander (OR, 0.66; 95% CI, 0.61-0.72) children were enrolled significantly less compared with the respective US populations of these groups. White children were enrolled less than expected (OR, 0.84; 95% CI, 0.84-0.85) but represented 188 156 (46.0%) of participants in trials reporting race or ethnicity.
Conclusions and relevance: This cross-sectional study revealed that the proportion of published pediatric clinical trials that reported participant race and ethnicity increased from 2011 to 2020, but participant race and ethnicity were still underreported. Disparities existed in pediatric clinical trial enrollment of American Indian/Alaska Native, Asian, and Native Hawaiian/Pacific Islander children. The greater representation of Black/African American children compared with the US population suggests inclusive research practices that could be extended to other historically disenfranchised racial and ethnic groups.
Conflict of interest statement
Figures

Comment in
-
Increasing Meaningful Representation of Children in Clinical Trials to Inform Science and Achieve Health Equity.JAMA Pediatr. 2022 May 1;176(5):e220139. doi: 10.1001/jamapediatrics.2022.0139. Epub 2022 May 2. JAMA Pediatr. 2022. PMID: 35311948 No abstract available.
References
-
- Hartling L, Scott-Findlay S, Johnson D, et al. ; Canadian Institutes for Health Research Team in Pediatric Emergency Medicine . Bridging the gap between clinical research and knowledge translation in pediatric emergency medicine. Acad Emerg Med. 2007;14(11):968-977. doi: 10.1197/j.aem.2007.05.010 - DOI - PubMed

