Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis: A Meta-analysis
- PMID: 35311963
- PMCID: PMC8938718
- DOI: 10.1001/jamanetworkopen.2022.3079
Comparison of Trials Using Ivermectin for COVID-19 Between Regions With High and Low Prevalence of Strongyloidiasis: A Meta-analysis
Abstract
Importance: A widely cited meta-analysis of randomized clinical trials has claimed ivermectin as an effective treatment for prevention of mortality in COVID-19. However, an unrecognized interaction variable with the relative risk (RR) of mortality may substantially change the appropriate interpretation of this analysis.
Objective: To evaluate the association between regional prevalence of strongyloidiasis and ivermectin trial results for the outcome of mortality by testing the hypothesis that strongyloidiasis prevalence interacts with the RR of mortality.
Data sources: Original meta-analysis as well as a manual review of all references in a dedicated ivermectin trial database (c19ivermectin) from January 1, 2019, to November 6, 2021.
Study selection: Randomized clinical trials using ivermectin as a treatment for COVID-19 and reporting the outcome of mortality. Studies were excluded in the event of publications revealing suspected trial fraud and/or randomization failure.
Data extraction and synthesis: Study characteristics and RR estimates were extracted from each source. Estimates were pooled using random-effects meta-analysis. Differences by strongyloidiasis prevalence were estimated using subgroup meta-analysis and meta-regression. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline was followed.
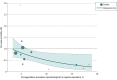
Main outcomes and measures: Relative risk of mortality in ivermectin trials in regions of high vs low strongyloidiasis prevalence and correlation coefficient of meta-regression analysis between RR of mortality and regional prevalence of strongyloidiasis.
Results: A total of 12 trials comprising 3901 patients were included in the analysis. Four trials (33%) took place in regions of high strongyloidiasis prevalence and 8 (67%) trials took place in regions of low strongyloidiasis prevalence. Ivermectin trials that took place in areas of low regional strongyloidiasis prevalence were not associated with a statistically significant decreased risk of mortality (RR, 0.84 [95% CI, 0.60-1.18]; P = .31). By contrast, ivermectin trials that took place in areas of high regional strongyloidiasis prevalence were associated with a significantly decreased risk of mortality (RR, 0.25 [95% CI, 0.09-0.70]; P = .008). Testing for subgroup differences revealed a significant difference between the results of groups with low and high strongyloidiasis prevalence (χ21 = 4.79; P = .03). The estimate for τ2 (the variance of the study effect sizes) was 0 (95% CI, 0.0000-0.2786), and the estimate for I2 (percentage of variability that is explained by between-study heterogeneity) was 0 (95% CI, 0-43.7%). The meta-regression analysis revealed an RR decrease of 38.83% (95% CI, 0.87%-62.25%) for each 5% increase in strongyloidiasis prevalence.
Conclusions and relevance: In this meta-analysis of 12 trials including 3901 patients, strongyloidiasis prevalence was found to interact with the RR of mortality for ivermectin as a treatment for COVID-19. No evidence was found to suggest ivermectin has any role in preventing mortality among patients with COVID-19 in regions where strongyloidiasis was not endemic.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

