N-terminal and mid-region tau fragments as fluid biomarkers in neurological diseases
- PMID: 35311972
- PMCID: PMC9420020
- DOI: 10.1093/brain/awab481
N-terminal and mid-region tau fragments as fluid biomarkers in neurological diseases
Abstract
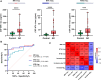
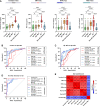
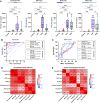
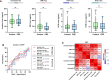
Brain-derived tau secreted into CSF and blood consists of different N-terminal and mid-domain fragments, which may have a differential temporal course and thus, biomarker potential across the Alzheimer's disease continuum or in other neurological diseases. While current clinically validated total tau assays target mid-domain epitopes, comparison of these assays with new biomarkers targeting N-terminal epitopes using the same analytical platform may be important to increase the understanding of tau pathophysiology. We developed three total tau immunoassays targeting specific N-terminal (NTA and NTB total tau) or mid-region (MR total tau) epitopes, using single molecule array technology. After analytical validation, the diagnostic performance of these biomarkers was evaluated in CSF and compared with the Innotest total tau (and as proof of concept, with N-p-tau181 and N-p-tau217) in three clinical cohorts (n = 342 total). The cohorts included participants across the Alzheimer's disease continuum (n = 276), other dementias (n = 22), Creutzfeldt-Jakob disease (n = 24), acute neurological disorders (n = 18) and progressive supranuclear palsy (n = 22). Furthermore, we evaluated all three new total tau biomarkers in plasma (n = 44) and replicated promising findings with NTA total tau in another clinical cohort (n = 50). In CSF, all total tau biomarkers were increased in Alzheimer's disease compared with controls (P < 0.0001) and correlated with each other (rs = 0.53-0.95). NTA and NTB total tau, but not other total tau assays, distinguished amyloid-positive and amyloid-negative mild cognitive impairment with high accuracies (AUCs 84% and 82%, P < 0.001) matching N-p-tau217 (AUC 83%; DeLong test P = 0.93 and 0.88). All total tau assays were excellent in differentiating Alzheimer's disease from other dementias (P < 0.001, AUCs 89-100%). In Creutzfeldt-Jakob disease and acute neurological disorders, N-terminal total tau biomarkers had significantly higher fold changes versus controls in CSF (45-133-fold increase) than Innotest or MR total tau (11-42-fold increase, P < 0.0001 for all). In progressive supranuclear palsy, CSF concentrations of all total tau biomarkers were similar to those in controls. Plasma NTA total tau concentrations were increased in Alzheimer's disease compared with controls in two independent cohorts (P = 0.0056 and 0.0033), while Quanterix total tau performed poorly (P = 0.55 and 0.44). Taken together, N-terminal-directed CSF total tau biomarkers increase ahead of standard total tau alternatives in the Alzheimer's disease continuum, increase to higher degrees in Creutzfeldt-Jakob disease and acute neurological diseases and show better potential than Quanterix total tau as Alzheimer's disease blood biomarkers. For progressive supranuclear palsy, other tau biomarkers should continue to be investigated.
Keywords: Alzheimer’s disease; biomarker; cerebrospinal fluid; plasma; tau.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. - PubMed
-
- Blennow K, Wallin A, Ågren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26(3):231–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

