Molecular contribution to embryonic aneuploidy and karyotypic complexity in initial cleavage divisions of mammalian development
- PMID: 35311995
- PMCID: PMC9058497
- DOI: 10.1242/dev.198341
Molecular contribution to embryonic aneuploidy and karyotypic complexity in initial cleavage divisions of mammalian development
Abstract
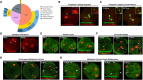
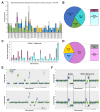
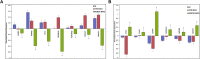
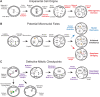
Embryonic aneuploidy is highly complex, often leading to developmental arrest, implantation failure or spontaneous miscarriage in both natural and assisted reproduction. Despite our knowledge of mitotic mis-segregation in somatic cells, the molecular pathways regulating chromosome fidelity during the error-prone cleavage-stage of mammalian embryogenesis remain largely undefined. Using bovine embryos and live-cell fluorescent imaging, we observed frequent micro-/multi-nucleation of mis-segregated chromosomes in initial mitotic divisions that underwent unilateral inheritance, re-fused with the primary nucleus or formed a chromatin bridge with neighboring cells. A correlation between a lack of syngamy, multipolar divisions and asymmetric genome partitioning was also revealed, and single-cell DNA-seq showed propagation of primarily non-reciprocal mitotic errors. Depletion of the mitotic checkpoint protein BUB1B (also known as BUBR1) resulted in similarly abnormal nuclear structures and cell divisions, as well as chaotic aneuploidy and dysregulation of the kinase-substrate network that mediates mitotic progression, all before zygotic genome activation. This demonstrates that embryonic micronuclei sustain multiple fates, provides an explanation for blastomeres with uniparental origins, and substantiates defective checkpoints and likely other maternally derived factors as major contributors to the karyotypic complexity afflicting mammalian preimplantation development.
Keywords: Aneuploidy; BUB1B; BUBR1; Cytokinesis; Embryo; Micronuclei; Mitosis.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Baker, D. J., Jeganathan, K. B., Cameron, J. D., Thompson, M., Juneja, S., Kopecka, A., Kumar, R., Jenkins, R. B., de Groen, P. C., Roche, P.et al. (2004). BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744-749. 10.1038/ng1382 - DOI - PubMed
-
- Baker, D. J., Dawlaty, M. M., Wijshake, T., Jeganathan, K. B., Malureanu, L., van Ree, J. H., Crespo-Diaz, R., Reyes, S., Seaburg, L., Shapiro, V.et al. (2013). Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 15, 96-102. 10.1038/ncb2643 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

