Pre-incubation conditions determine the fermentation pattern and microbial community structure in fermenters at mild hydrostatic pressure
- PMID: 35312065
- PMCID: PMC9325544
- DOI: 10.1002/bit.28085
Pre-incubation conditions determine the fermentation pattern and microbial community structure in fermenters at mild hydrostatic pressure
Abstract
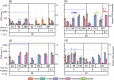
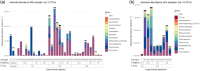
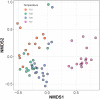
Fermentation at elevated hydrostatic pressure is a novel strategy targeting product selectivity. However, the role of inoculum history and cross-resistance, that is, acquired tolerance from incubation under distinctive environmental stress, remains unclear in high-pressure operation. In our here presented work, we studied fermentation and microbial community responses of halotolerant marine sediment inoculum (MSI) and anaerobic digester inoculum (ADI), pre-incubated in serum bottles at different temperatures and subsequently exposed to mild hydrostatic pressure (MHP; < 10 MPa) in stainless steel reactors. Results showed that MHP effects on microbial growth, activity, and community structure were strongly temperature-dependent. At moderate temperature (20°C), biomass yield and fermentation were not limited by MHP; suggesting a cross-resistance effect from incubation temperature and halotolerance. Low temperatures (10°C) and MHP imposed kinetic and bioenergetic limitations, constraining growth and product formation. Fermentation remained favorable in MSI at 28°C and ADI at 37°C, despite reduced biomass yield resulting from maintenance and decay proportionally increasing with temperature. Microbial community structure was modified by temperature during the enrichment, and slight differences observed after MHP-exposure did not compromise functionality. Results showed that the relation incubation temperature-halotolerance proved to be a modifier of microbial responses to MHP and could be potentially exploited in fermentations to modulate product/biomass ratio.
Keywords: anaerobic fermentation; halotolerance; mild hydrostatic pressure; piezotolerance; psychrotolerance.
© 2022 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Pressure and temperature effects on deep-sea hydrocarbon-degrading microbial communities in subarctic sediments.Microbiologyopen. 2019 Jun;8(6):e00768. doi: 10.1002/mbo3.768. Epub 2018 Nov 16. Microbiologyopen. 2019. PMID: 30444300 Free PMC article.
-
Inactivation of Shiga toxin-producing Escherichia coli O157: H7 and mesophilic background microbiota of meat homogenate using elevated hydrostatic pressure, mild heat, and thymol.J Food Sci. 2020 Dec;85(12):4335-4341. doi: 10.1111/1750-3841.15526. Epub 2020 Nov 14. J Food Sci. 2020. PMID: 33190218
-
Effect of pressure and temperature on anaerobic methanotrophic activities of a highly enriched ANME-2a community.Environ Sci Pollut Res Int. 2018 Oct;25(30):30031-30043. doi: 10.1007/s11356-018-2573-2. Epub 2018 Jun 26. Environ Sci Pollut Res Int. 2018. PMID: 29946835
-
Combined effect of pressure and temperature for yogurt production.Food Res Int. 2019 Aug;122:222-229. doi: 10.1016/j.foodres.2019.04.010. Epub 2019 Apr 8. Food Res Int. 2019. PMID: 31229075 Review.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
References
-
- American Public Health Association . (2017). Standard methods for the examination of water and wastewater (23rd ed.).
-
- Arnosti, C. , Jørgensen, B. , Sagemann, J. , & Thamdrup, B. (1998). Temperature dependence of microbial degradation of organic matter in marine sediments: Polysaccharide hydrolysis, oxygen consumption, and sulfate reduction. Marine Ecology Progress Series, 165, 59–70. 10.3354/meps165059 - DOI
-
- Arslan, D. , Steinbusch, K. J. J. , Diels, L. , Hamelers, H. V. M. , Strik, D. P. B. T. B. , Buisman, C. J. N. , & De Wever, H. (2016). Selective short‐chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Critical Reviews in Environmental Science and Technology, 46(6), 592–634. 10.1080/10643389.2016.1145959 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

