Time to diagnosis of Duchenne muscular dystrophy remains unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015
- PMID: 35312090
- PMCID: PMC9308714
- DOI: 10.1002/mus.27532
Time to diagnosis of Duchenne muscular dystrophy remains unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015
Abstract
Introduction/aims: With current and anticipated disease-modifying treatments, including gene therapy, an early diagnosis for Duchenne muscular dystrophy (DMD) is crucial to assure maximum benefit. In 2009, a study from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) showed an average diagnosis age of 5 years among males with DMD born from January 1, 1982 to December 31, 2000. Initiatives were implemented by the US Centers for Disease Control and Prevention (CDC) and patient organizations to reduce time to diagnosis. We conducted a follow-up study in a surveillance cohort born after January 1, 2000 to determine whether there has been an improvement in time to diagnosis.
Methods: We assessed the age of diagnosis among males with DMD born from January 1, 2000 to December 31, 2015 using data collected by six US MD STARnet surveillance sites (Colorado, Iowa, western New York State, the Piedmont region of North Carolina, South Carolina, and Utah). The analytic cohort included 221 males with definite or probable DMD diagnosis without a documented family history. We computed frequency count and percentage for categorical variables, and mean, median, and standard deviation (SD) for continuous variables.
Results: The mean [median] ages in years of diagnostic milestones were: first signs, 2.7 [2.0]; first creatine kinase (CK), 4.6 [4.6]; DNA/muscle biopsy testing, 4.9 [4.8]; and time from first signs to diagnostic confirmation, 2.2 [1.4].
Discussion: The time interval between first signs of DMD and diagnosis remains unchanged at 2.2 years. This results in lost opportunities for timely genetic counseling, implementation of standards of care, initiation of glucocorticoids, and participation in clinical trials.
Keywords: Duchenne muscular dystrophy; MD STARnet; delay; diagnostic criteria; muscle dystrophy; surveillance.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
Dr. Emma Ciafaloni has received personal compensation for serving on advisory boards and/or as a consultant for Viela Bio, Avexis, Biogen, Medscape, Amicus, PTC Therapeutics, Sarepta Therapeutics, Ra Pharma, Wave, and Strongbridge Biopharma plc. Dr. Ciafaloni has received personal compensation for serving on a speaker’s bureau for Biogen as well as research and/or grant support from the CDC, CureSMA, Muscular Dystrophy Association, National Institutes of Health, Orphazyme, the Patient-Centered Outcomes Research Institute, Parent Project Muscular Dystrophy, PTC Therapeutics, Santhera, Sarepta Therapeutics, Orphazyme, and the US Food and Drug Administration. Dr. Ciafaloni has also received royalties from Oxford University Press and compensation from
Dr. Katherine Mathews receives research funding from NIH (NIAMS) P50 NS053672, NIH (NINDS) U24 NS-107181, and the Friedreich’s Ataxia Research Alliance, and serves as a site PI for clinical research sponsored by Italfarmaco, PTC, Sarepta, Pfizer, Retrotope, Reata and AMO.
The other authors declare no conflicts of interest.
Figures
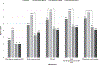
Comment in
-
Age at diagnosis for Duchenne muscular dystrophy: Why we must do better.Muscle Nerve. 2022 Aug;66(2):116-117. doi: 10.1002/mus.27574. Epub 2022 Jun 6. Muscle Nerve. 2022. PMID: 35560237 No abstract available.
References
Publication types
MeSH terms
Grants and funding
- U01DD001120/ACL/ACL HHS/United States
- U01DD001054/ACL/ACL HHS/United States
- U01 DD001123/DD/NCBDD CDC HHS/United States
- U01DD001126/ACL/ACL HHS/United States
- U01 DD001116/DD/NCBDD CDC HHS/United States
- U24 NS107181/NS/NINDS NIH HHS/United States
- U01DD001108/ACL/ACL HHS/United States
- U01 DD001120/DD/NCBDD CDC HHS/United States
- P50 NS053672/NS/NINDS NIH HHS/United States
- U01 DD001117/DD/NCBDD CDC HHS/United States
- U01 DD001126/DD/NCBDD CDC HHS/United States
- U01DD001116/ACL/ACL HHS/United States
- U01 DD001247/DD/NCBDD CDC HHS/United States
- U01DD001119/ACL/ACL HHS/United States
- U01DD001123/ACL/ACL HHS/United States
- U01 DD001119/DD/NCBDD CDC HHS/United States
- U01 DD001108/DD/NCBDD CDC HHS/United States
- U01 DD001248/DD/NCBDD CDC HHS/United States
- U01 DD001054/DD/NCBDD CDC HHS/United States
- U01DD001117/ACL/ACL HHS/United States
- CC999999/ImCDC/Intramural CDC HHS/United States
- U01 DD001250/DD/NCBDD CDC HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

