Establishment of an in-house real-time RT-PCR assay for the detection of severe acute respiratory syndrome coronavirus 2 using the first World Health Organization international standard in a resource-limited country
- PMID: 35312118
- PMCID: PMC9102516
- DOI: 10.1002/jcla.24355
Establishment of an in-house real-time RT-PCR assay for the detection of severe acute respiratory syndrome coronavirus 2 using the first World Health Organization international standard in a resource-limited country
Abstract
Background: The COVID-19 pandemic caused by SARS-CoV-2 remains public health burdens and many unresolved issues worldwide. Molecular assays based on real-time RT-PCR are critical for the detection of SARS-CoV-2 in clinical specimens from patients suspected of COVID-19.
Objective: We aimed to establish and validate an in-house real-time RT-PCR for the detection of SARS-CoV-2.
Methodology: Primers and probes sets in our in-house real-time RT-PCR assay were designed in conserved regions of the N and E target genes. Optimized multiplex real-time RT-PCR assay was validated using the first WHO International Standard (NIBSC code: 20/146) and evaluated clinical performance.
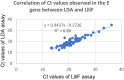
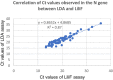
Results: The limit of detection validated using the first WHO International Standard was 159 IU/ml for both E and N target genes. The evaluation of clinical performance on 170 clinical samples showed a positive percent agreement of 100% and the negative percent agreement of 99.08% for both target genes. The Kappa value of 0.99 was an excellent agreement, the strong correlation of Ct values observed between two tests with r2 = 0.84 for the E gene and 0.87 for the N gene. Notably, we assessed on 60 paired saliva and nasopharyngeal samples. The overall agreement was 91.66%, and Kappa value of 0.74 showed a high agreement between two types of samples. When using nasopharyngeal swabs as the reference standard, positive percent agreement, and negative percent agreement were 91.83% and 90.90%, respectively.
Conclusion: In the present study, we established and validated an in-house real-time RT-PCR for molecular detection of SARS-CoV-2 in a resource-limited country.
Keywords: SARS-CoV-2; clinical performance; real-time RT-PCR.
© 2022 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Weekly epidemiological update on . COVID‐19 ‐ 7 December 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on... Accessed December 15, 2021.
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819‐1829. doi:10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

