Functional enzyme-polymer complexes
- PMID: 35312375
- PMCID: PMC9060439
- DOI: 10.1073/pnas.2119509119
Functional enzyme-polymer complexes
Abstract
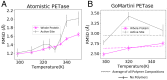
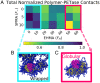
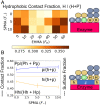
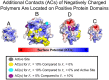
SignificanceThe use of biological enzyme catalysts could have huge ramifications for chemical industries. However, these enzymes are often inactive in nonbiological conditions, such as high temperatures, present in industrial settings. Here, we show that the enzyme PETase (polyethylene terephthalate [PET]), with potential application in plastic recycling, is stabilized at elevated temperature through complexation with random copolymers. We demonstrate this through simulations and experiments on different types of substrates. Our simulations also provide strategies for designing more enzymatically active complexes by altering polymer composition and enzyme charge distribution.
Keywords: GoMartini; coarse-grained molecular simulations; complex coacervation; enzymes; random copolymers.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Coon M. J., Cytochrome P450: Nature’s most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 45, 1–25 (2005). - PubMed
-
- Martinez C. A., Rupashinghe S. G., Cytochrome P450 bioreactors in the pharmaceutical industry: Challenges and opportunities. Curr. Top. Med. Chem. 13, 1470–1490 (2013). - PubMed
-
- Sureka H. V., Obermeyer A. C., Flores R. J., Olsen B. D., Catalytic biosensors from complex coacervate core micelle (c3m) thin films. ACS Appl. Mater. Interfaces 11, 32354–32365 (2019). - PubMed
-
- DelRe C., et al. ., Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 592, 558–563 (2021). - PubMed
-
- Blank L. M., Narancic T., Mampel J., Tiso T., O’Connor K., Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 62, 212–219 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

