Cost-Effectiveness of Hepatitis B Testing and Vaccination of Adults Seeking Care for Sexually Transmitted Infections
- PMID: 35312661
- PMCID: PMC9188991
- DOI: 10.1097/OLQ.0000000000001632
Cost-Effectiveness of Hepatitis B Testing and Vaccination of Adults Seeking Care for Sexually Transmitted Infections
Abstract
Background: The estimated number of people living with hepatitis B virus (HBV) infection acquired through sexual transmission was 103,000 in 2018, with an estimated incidence of 8300 new cases per year. Although hepatitis B (HepB) vaccination is recommended by the Advisory Committee for Immunization Practices for persons seeking evaluation and treatment for sexually transmitted infections (STIs), prevaccination testing is not yet recommended. Screening may link persons with chronic hepatitis B to care and reduce unnecessary vaccination.
Methods: We used a Markov model to calculate the health impact and cost-effectiveness of 1-time HBV testing combined with the first dose of the HepB vaccine for adults seeking care for STI. We ran a lifetime, societal perspective analysis for a hypothetical population of 100,000 aged 18 to 69 years. The disease progression estimates were taken from recent cohort studies and meta-analyses. In the United States, an intervention that costs less than $100,000 per quality-adjusted life-year (QALY) is generally considered cost-effective. The strategies that were compared were as follows: (1) vaccination without HBV screening, (2) vaccination and hepatitis B surface antigen (HBsAg) screening, (3) vaccination and screening with HBsAg and anti-HBs, and (4) vaccination and screening with HBsAg, anti-HBs, and anti-HBc. Data were obtained from Centers for Medicare & Medicaid services reimbursement, the Centers for Disease Control and Prevention vaccine price list, and additional cost-effectiveness literature.
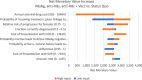
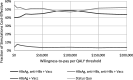
Results: Compared with current recommendations, the addition of 1-time HBV testing is cost-saving and would prevent an additional 138 cases of cirrhosis, 47 cases of decompensated cirrhosis, 90 cases of hepatocellular carcinoma, 33 liver transplants, and 163 HBV-related deaths, and gain 2185 QALYs, per 100,000 adults screened. Screening with the 3-test panel would save $41.6 to $42.7 million per 100,000 adults tested compared with $41.5 to $42.5 million for the 2-test panel and $40.2 to $40.3 million for HBsAg alone.
Conclusions: One-time HBV prevaccination testing in addition to HepB vaccination for unvaccinated adults seeking care for STI would save lives and prevent new infections and unnecessary vaccination, and is cost-saving.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Sexually Transmitted Diseases Association.
Conflict of interest statement
Conflict of Interest and Sources of Funding: The authors have no conflict of interest to declare. This work was supported by The US Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NU38PS004651).
Figures

References
-
- Toy M Hutton D Harris AM, et al. . Cost-effectiveness of one-time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis 2022; 74:210–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

