From Deer-to-Deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer
- PMID: 35312736
- PMCID: PMC8970504
- DOI: 10.1371/journal.ppat.1010197
From Deer-to-Deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer
Abstract
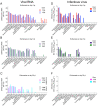
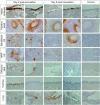
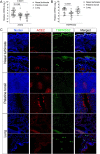
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19) in humans, has a broad host range, and is able to infect domestic and wild animal species. Notably, white-tailed deer (WTD, Odocoileus virginianus), the most widely distributed cervid species in the Americas, were shown to be highly susceptible to SARS-CoV-2 in challenge studies and reported natural infection/exposure rates approaching 30-40% in free-ranging WTD in the U.S. Thus, understanding the infection and transmission dynamics of SARS-CoV-2 in WTD is critical to prevent future zoonotic transmission to humans, at the human-WTD interface during hunting or venison farming, and for implementation of effective disease control measures. Here, we demonstrated that following intranasal inoculation with SARS-CoV-2 B.1 lineage, WTD fawns (~8-month-old) shed infectious virus up to day 5 post-inoculation (pi), with high viral loads shed in nasal and oral secretions. This resulted in efficient deer-to-deer transmission on day 3 pi. Consistent a with lack of infectious SARS-CoV-2 shedding after day 5 pi, no transmission was observed to contact animals added on days 6 and 9 pi. We have also investigated the tropism and sites of SARS-CoV-2 replication in adult WTD (3-4 years of age). Infectious virus was detected up to day 6 pi in nasal secretions, and from various respiratory-, lymphoid-, and central nervous system tissues, indicating broad tissue tropism and multiple sites of virus replication. The study provides important insights on the infection and transmission dynamics of SARS-CoV-2 in WTD, a wild animal species that is highly susceptible to infection and with the potential to become a reservoir for the virus in the field.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al.. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. Nature Research; 2020. pp. 536–544. doi: 10.1038/s41564-020-0695-z - DOI - PMC - PubMed
-
- Temmam S, Vongphayloth K, Salazar EB, Munier S, Bonomi M, Régnault B, et al.. Coronaviruses with a SARS-CoV-2-like receptor- binding domain allowing ACE2-mediated entry into human cells isolated from bats of Indochinese peninsula. Res Sq. 2021.
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

