The use of photobiomodulation therapy for the prevention of chemotherapy-induced peripheral neuropathy: a randomized, placebo-controlled pilot trial (NEUROLASER trial)
- PMID: 35312857
- PMCID: PMC8935622
- DOI: 10.1007/s00520-022-06975-x
The use of photobiomodulation therapy for the prevention of chemotherapy-induced peripheral neuropathy: a randomized, placebo-controlled pilot trial (NEUROLASER trial)
Abstract
Purpose: The purpose of this study was to investigate the effectiveness of photobiomodulation (PBM) therapy for the prevention of chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer patients.
Methods: A prospective, randomized placebo-controlled pilot trial (NEUROLASER) was set up with 32 breast cancer patients who underwent chemotherapy (ClinicalTrials.gov; NCT03391271). Patients were randomized to receive PBM (n = 16) or placebo treatments (n = 16) (2 × /week) during their chemotherapy. The modified Total Neuropathy Score (mTNS), six-minute walk test (6MWT), Numeric pain Rating Scale (NRS), and Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Taxane (FACT/GOG-Taxane) were used to evaluate the severity of CIPN and the patients' quality of life (QoL). Outcome measures were collected at the first chemotherapy session, 6 weeks after initiation of chemotherapy, at the final chemotherapy session, and 3 weeks after the end of chemotherapy (follow-up).
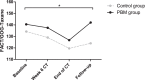
Results: The mTNS score increased significantly over time in both the control and the PBM group. A significantly higher score for FACT/GOG-Taxane was observed in the PBM group during chemotherapy compared to the control group. Questions of the FACT/GOG-Taxane related to sensory peripheral neuropathy symptoms showed a significant increase in severeness over time in the control group, whereas they remained constant in the PBM group. At follow-up, a (borderline) significant difference was observed between both groups for the 6MWT and patients' pain level, in benefit of the PBM group.
Conclusions: This NEUROLASER trial shows promising results concerning the prevention of CIPN with PBM in breast cancer patients. Furthermore, a better QoL was observed when treated with PBM.
Keywords: Breast cancer; Chemotherapy; Peripheral neuropathy; Photobiomodulation; Polyneuropathy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hou S, et al. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician. 2018;21(6):571–592. - PubMed
-
- Mols F, et al. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22(8):2261–2269. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

