Improvement in disease activity among patients with rheumatoid arthritis who switched from intravenous infliximab to intravenous golimumab in the ACR RISE registry
- PMID: 35312895
- PMCID: PMC9287251
- DOI: 10.1007/s10067-022-06116-z
Improvement in disease activity among patients with rheumatoid arthritis who switched from intravenous infliximab to intravenous golimumab in the ACR RISE registry
Abstract
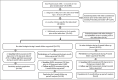
Infliximab and golimumab are intravenously (IV) administered tumor necrosis factor inhibitors approved to treat moderate-to-severe rheumatoid arthritis (RA) with concomitant methotrexate. Owing to differences in biologic construct, patients with IV-infliximab treatment failure may benefit from switching to IV-golimumab. Utilizing the ACR's Rheumatology Informatics System for Effectiveness (RISE), a large electronic health records registry based in the USA, we assessed RA disease activity in patients switching from IV-infliximab to IV-golimumab. This retrospective, longitudinal, single-arm study included adults (≥ 18 years) with ≥ 1 RA diagnosis code between 2014 and 2018 and ≥ 1 IV-infliximab prescription within 6 months of a new IV-golimumab order (index date). Longitudinal assessments of disease activity using the Clinical Disease Activity Index (CDAI) were calculated in patients continuing IV-golimumab for 6-9- and 9-12-months post-switch. Paired t-tests evaluated significance of mean improvements during the follow-up periods. Most RA patients with disease activity assessments during the 6-month follow-up (N = 100; mean age: 65.3 years; 81% female; 74% white) demonstrated moderate-to-high disease activity (CDAI: 73% [38/52]) at enrollment. On average, patients showed significant improvement in disease activity within 6-9 months of switching; mean CDAI scores improved from 21.3 to 14.1 (p < 0.0001) and were durable through 9-12 months of treatment. Real-world patients with moderate-to-high disease activity who switched from IV-infliximab to IV-golimumab demonstrated significant and sustained improvements post-switch as measured by the CDAI. Key Points • This study used real-world data from the Rheumatology Informatics System for Effectiveness (RISE) registry to evaluate the efficacy of directly switching from intravenous (IV)-infliximab to IV-golimumab to control rheumatoid arthritis (RA) disease activity. • Most IV-infliximab patients had moderate-to-high disease activity at the time of the switch. • On average, IV-golimumab was effective in improving RA disease activity after switching from IV-infliximab as measured by the Clinical Disease Activity Index. • These data suggest that real-world RA patients with persistent symptoms despite treatment with IV-infliximab may realize improved disease control with a switch to IV-golimumab.
Keywords: Disease activity; Intravenous golimumab; Intravenous infliximab; Registry; Rheumatoid arthritis; Tumor necrosis factor.
© 2022. The Author(s).
Conflict of interest statement
JT has served on advisory boards/as a consultant for AbbVie, AstraZeneca, Aurinia, Bristol Myers Squibb, Janssen, Eli Lilly, Novartis, Pfizer, and Sanofi-Genzyme; served as a speaker for AbbVie, Amgen, AstraZeneca, Aurinia, Bristol Myers Squibb, Eli Lilly, Genentech, GSK, Janssen, Pfizer, and Sanofi-Genzyme; and received research grants and support from AbbVie, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, CSL Behring, Eli Lilly, Genentech, Gilead, GlaxoSmithKline, Horizon, Janssen, Merck KG, Novartis, Pfizer, R-Pharma, Regeneron, Roche, Sandoz, Scipher, Takeda, Sun Pharma, UCB, Selecta, and Vorso. IL, NS, and SDC are employees of Janssen Scientific Affairs, LLC, and own stock in Johnson & Johnson, of which Janssen Scientific Affairs, LLC, is a wholly owned subsidiary of (IL, NS, SDC), AbbVie (NS), and Gilead (NS). GS is funded by AHRQ and VA HSR&D. NH has no disclosures. SD served on speakers bureaus for AstraZeneca, Aurinia, GlaxoSmithKline, and Janssen.
Figures

Similar articles
-
The Effectiveness of Intravenous Golimumab Administered Directly After Infliximab in Rheumatoid Arthritis Patients.Drugs R D. 2018 Sep;18(3):211-219. doi: 10.1007/s40268-018-0240-1. Drugs R D. 2018. PMID: 30054896 Free PMC article.
-
Characteristics and 6-Month Outcomes in Patients with Rheumatoid Arthritis Initiating Infliximab Biosimilar IFX-dyyb in a Real-World Setting.Rheumatol Ther. 2024 Jun;11(3):841-853. doi: 10.1007/s40744-024-00653-6. Epub 2024 Mar 20. Rheumatol Ther. 2024. PMID: 38507187 Free PMC article.
-
Comparative Assessment of the Different American College of Rheumatology/European League Against Rheumatism Remission Definitions for Rheumatoid Arthritis for Their Use as Clinical Trial End Points.Arthritis Rheumatol. 2017 Mar;69(3):518-528. doi: 10.1002/art.39945. Arthritis Rheumatol. 2017. PMID: 27696724
-
Re-Routing Infliximab Therapy: Subcutaneous Infliximab Opens a Path Towards Greater Convenience and Clinical Benefit.Clin Drug Investig. 2022 Jun;42(6):477-489. doi: 10.1007/s40261-022-01162-6. Epub 2022 Jun 3. Clin Drug Investig. 2022. PMID: 35657560 Review.
-
Infliximab: an updated review of its use in Crohn's disease and rheumatoid arthritis.BioDrugs. 2002;16(2):111-48. doi: 10.2165/00063030-200216020-00005. BioDrugs. 2002. PMID: 11985485 Review.
References
-
- Simponi: Package Insert (2019) https://www.janssenlabels.com/package-insert/product-monograph/prescribi.... Accessed 4 Dec 2021
-
- Simponi ARIA: Package Insert (2021) https://www.janssenlabels.com/package-insert/product-monograph/prescribi.... Accessed 4 Dec 2021
-
- Remicade: Package Insert (2013) https://www.janssenlabels.com/package-insert/product-monograph/prescribi.... Accessed 4 Dec 2021
-
- Lipsky PE, van der Heijde DMFM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed

