Functioning of K channels during sleep
- PMID: 35313039
- PMCID: PMC9261471
- DOI: 10.1002/arch.21884
Functioning of K channels during sleep
Abstract
The functioning of voltage-dependent K channels (Kv) may correlate with the physiological state of brain in organisms, including the sleep in Drosophila. Apparently, all major types of K currents are expressed in CNS of this model organism. These are the Shab-Kv2, Shaker-Kv1, Shal-Kv4, and Shaw-Kv3 α subunits and can be deciphered by patch-clamp technique. Although it is plausible that some of these channels may play a prevailing role in sleep or wakefulness, several of recent data are not conclusive. It needs to be defined that indeed the frequency of action potentials in large ventral lateral pacemaker neurons is either higher or lower during the morning or night because of an increased Kv3 and Kv4 currents, respectively. The outcomes of dynamic-clamp approach in combination with electrophysiology in insects are unreliable in contrast to those in mammalian neurons. Since the addition of virtual Kv conductance during any Zeitgeber time should not significantly alter the resting membrane potential. This review explains the Drosophila sleep behavior based on neural activity with respect to K current-driven action potential rate.
Keywords: Kv1; Kv2; Kv3; Kv4; Patch-clamp; dynamic-clamp.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
Figures

 at dashed line indicate the 30 pA/pF level). LNV, large ventral lateral; RMP, resting membrane potential
at dashed line indicate the 30 pA/pF level). LNV, large ventral lateral; RMP, resting membrane potential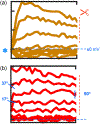
 symbol points to converging behavior of all traces. (b) Ordinary, the patch-clamp traces do not behave as presented for Shaw. If one chooses to present for “clarity” a certain portion of traces, the pClamp or any software truncates them at 90°, but not at 17°, 37°, and so forth. Even if it is performed by cut, copy, and paste, the angle is always 90°. Modified and highlighted (Smith et al., 2019)
symbol points to converging behavior of all traces. (b) Ordinary, the patch-clamp traces do not behave as presented for Shaw. If one chooses to present for “clarity” a certain portion of traces, the pClamp or any software truncates them at 90°, but not at 17°, 37°, and so forth. Even if it is performed by cut, copy, and paste, the angle is always 90°. Modified and highlighted (Smith et al., 2019)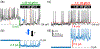
 depolarization with that of currents in (b). Dashed boxes reflect changes in spike amplitude. (b) Corresponding current behavior. Arrows point whether the MP and spikes or currents are drivers. (c) Perhaps the same neuron in the presence of Shaw, since the proximate level of RMP is identical at −54 mV. Now an opposite effect is observed on RMP, though it is ~1.4 mV depolarization. The effects of the same type of channels cannot have opposite polarities. The latter is perhaps derived by artificially assigning the negative polarity for “removed” Shaw as −1.4 nS in (a) and Shal as −1.25 nS in (c). (d) Current behavior either in response to or followed by MP and spikes in (c). Note that in contrast to (a and b) pair, the behavior of (c and d) one are almost 100% similar. LNV, large ventral lateral; MP, membrane potential; RMP, resting membrane potential
depolarization with that of currents in (b). Dashed boxes reflect changes in spike amplitude. (b) Corresponding current behavior. Arrows point whether the MP and spikes or currents are drivers. (c) Perhaps the same neuron in the presence of Shaw, since the proximate level of RMP is identical at −54 mV. Now an opposite effect is observed on RMP, though it is ~1.4 mV depolarization. The effects of the same type of channels cannot have opposite polarities. The latter is perhaps derived by artificially assigning the negative polarity for “removed” Shaw as −1.4 nS in (a) and Shal as −1.25 nS in (c). (d) Current behavior either in response to or followed by MP and spikes in (c). Note that in contrast to (a and b) pair, the behavior of (c and d) one are almost 100% similar. LNV, large ventral lateral; MP, membrane potential; RMP, resting membrane potential

 denotes one of hallmarks in both traces in space and time. Hyperpolarization is perhaps triggered by a “removed” Shaw (−1.4 nS), while depolarization by Shal (−1.25 nS) or else. If these currents are “outputs” then they cannot be identical. Otherwise, it is just a white noise, which is unable to alter the baseline spontaneous excitability and background MP fluctuations. Modified and highlighted (Smith et al., 2019). MP, membrane potential
denotes one of hallmarks in both traces in space and time. Hyperpolarization is perhaps triggered by a “removed” Shaw (−1.4 nS), while depolarization by Shal (−1.25 nS) or else. If these currents are “outputs” then they cannot be identical. Otherwise, it is just a white noise, which is unable to alter the baseline spontaneous excitability and background MP fluctuations. Modified and highlighted (Smith et al., 2019). MP, membrane potential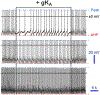
References
-
- Burdina EV, Bykov RA, Menshanov PN, Ilinsky YY, & Gruntenko NE (2021). Unique Wolbachia strain wMelPlus increases heat stress resistance in Drosophila melanogaster. Archives of Insect Biochemistry and Physiology, 106, e21776. - PubMed
-
- Clay JR (1985). Potassium current in the squid giant axon. International Review Neurobiology, 27, 363–384. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

