High BMP4 expression in low/intermediate risk BCP-ALL identifies children with poor outcomes
- PMID: 35313334
- PMCID: PMC11022983
- DOI: 10.1182/blood.2021013506
High BMP4 expression in low/intermediate risk BCP-ALL identifies children with poor outcomes
Abstract
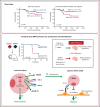
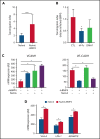
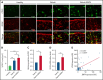
Pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL) outcome has improved in the last decades, but leukemic relapses are still one of the main problems of this disease. Bone morphogenetic protein 4 (BMP4) was investigated as a new candidate biomarker with potential prognostic relevance, and its pathogenic role was assessed in the development of disease. A retrospective study was performed with 115 pediatric patients with BCP-ALL, and BMP4 expression was analyzed by quantitative reverse transcription polymerase chain reaction in leukemic blasts at the time of diagnosis. BMP4 mRNA expression levels in the third (upper) quartile were associated with a higher cumulative incidence of relapse as well as a worse 5-year event-free survival and central nervous system (CNS) involvement. Importantly, this association was also evident among children classified as having a nonhigh risk of relapse. A validation cohort of 236 patients with BCP-ALL supported these data. Furthermore, high BMP4 expression promoted engraftment and rapid disease progression in an NSG mouse xenograft model with CNS involvement. Pharmacological blockade of the canonical BMP signaling pathway significantly decreased CNS infiltration and consistently resulted in amelioration of clinical parameters, including neurological score. Mechanistically, BMP4 favored chemoresistance, enhanced adhesion and migration through brain vascular endothelial cells, and promoted a proinflammatory microenvironment and CNS angiogenesis. These data provide evidence that BMP4 expression levels in leukemic cells could be a useful biomarker to identify children with poor outcomes in the low-/intermediate-risk groups of BCP-ALL and that BMP4 could be a new therapeutic target to blockade leukemic CNS disease.
© 2022 by The American Society of Hematology.
Figures



References
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. - PubMed
-
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. - PubMed
-
- Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. - PubMed
-
- Evans AE, Gilbert ES, Zandstra R. The increasing incidence of central nervous system leukemia in children. (Children's Cancer Study Group A) Cancer. 1970;26(2):404–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

