Acute intermittent hypoxia drives hepatic de novo lipogenesis in humans and rodents
- PMID: 35313531
- PMCID: PMC8933516
- DOI: 10.1016/j.metop.2022.100177
Acute intermittent hypoxia drives hepatic de novo lipogenesis in humans and rodents
Abstract
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver condition. It is tightly associated with an adverse metabolic phenotype (including obesity and type 2 diabetes) as well as with obstructive sleep apnoea (OSA) of which intermittent hypoxia is a critical component. Hepatic de novo lipogenesis (DNL) is a significant contributor to hepatic lipid content and the pathogenesis of NAFLD and has been proposed as a key pathway to target in the development of pharmacotherapies to treat NAFLD. Our aim is to use experimental models to investigate the impact of hypoxia on hepatic lipid metabolism independent of obesity and metabolic disease.
Methods: Human and rodent studies incorporating stable isotopes and hyperinsulinaemic euglycaemic clamp studies were performed to assess the regulation of DNL and broader metabolic phenotype by intermittent hypoxia. Cell-based studies, including pharmacological and genetic manipulation of hypoxia-inducible factors (HIF), were used to examine the underlying mechanisms.
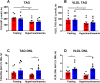
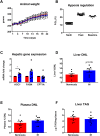
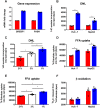
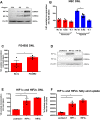
Results: Hepatic DNL increased in response to acute intermittent hypoxia in humans, without alteration in glucose production or disposal. These observations were endorsed in a prolonged model of intermittent hypoxia in rodents using stable isotopic assessment of lipid metabolism. Changes in DNL were paralleled by increases in hepatic gene expression of acetyl CoA carboxylase 1 and fatty acid synthase. In human hepatoma cell lines, hypoxia increased both DNL and fatty acid uptake through HIF-1α and -2α dependent mechanisms.
Conclusions: These studies provide robust evidence linking intermittent hypoxia and the regulation of DNL in both acute and sustained in vivo models of intermittent hypoxia, providing an important mechanistic link between hypoxia and NAFLD.
Keywords: HIF; Hypoxia; Lipid metabolism; NAFLD.
© 2022 The Authors.
Conflict of interest statement
None of the authors have any conflicts of interest or any relevant financial disclosures.
Figures

Similar articles
-
Tcf7l2 in hepatocytes regulates de novo lipogenesis in diet-induced non-alcoholic fatty liver disease in mice.Diabetologia. 2023 May;66(5):931-954. doi: 10.1007/s00125-023-05878-8. Epub 2023 Feb 10. Diabetologia. 2023. PMID: 36759348 Free PMC article.
-
Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease.J Clin Invest. 2020 Mar 2;130(3):1453-1460. doi: 10.1172/JCI134165. J Clin Invest. 2020. PMID: 31805015 Free PMC article. Clinical Trial.
-
Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease.Dig Dis Sci. 2016 May;61(5):1282-93. doi: 10.1007/s10620-016-4054-0. Epub 2016 Feb 8. Dig Dis Sci. 2016. PMID: 26856717 Free PMC article. Review.
-
Fructose as a key player in the development of fatty liver disease.World J Gastroenterol. 2013 Feb 28;19(8):1166-72. doi: 10.3748/wjg.v19.i8.1166. World J Gastroenterol. 2013. PMID: 23482247 Free PMC article. Review.
-
Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: Results from two early-phase randomized trials.Diabetes Obes Metab. 2021 Mar;23(3):700-710. doi: 10.1111/dom.14272. Epub 2020 Dec 21. Diabetes Obes Metab. 2021. PMID: 33289350 Free PMC article. Clinical Trial.
Cited by
-
Editorial: Special issue: Non-alcoholic fatty liver disease: From molecular basis to therapeutic advances.Metabol Open. 2023 Jan 13;17:100229. doi: 10.1016/j.metop.2023.100229. eCollection 2023 Mar. Metabol Open. 2023. PMID: 36686606 Free PMC article. No abstract available.
-
Childhood insulin resistance and neural stem cell dysfunction in psychiatric disorders: Role of de novo lipogenesis and treatment perspectives.World J Stem Cells. 2025 Jul 26;17(7):106194. doi: 10.4252/wjsc.v17.i7.106194. World J Stem Cells. 2025. PMID: 40740530 Free PMC article. Review.
-
The Effect of Acute Intermittent and Continuous Hypoxia on Plasma Circulating ßOHB Levels Under Different Feeding Statuses in Humans.Front Physiol. 2022 Jul 6;13:937127. doi: 10.3389/fphys.2022.937127. eCollection 2022. Front Physiol. 2022. PMID: 35874514 Free PMC article.
-
Duration of intermittent hypoxia impacts metabolic outcomes and severity of murine NAFLD.Front Sleep. 2023;2:1215944. doi: 10.3389/frsle.2023.1215944. Epub 2023 Aug 25. Front Sleep. 2023. PMID: 38077744 Free PMC article.
-
Chronic intermittent hypoxia due to obstructive sleep apnea slightly alters nutritional status: a pre-clinical study.Front Nutr. 2023 Oct 30;10:1250529. doi: 10.3389/fnut.2023.1250529. eCollection 2023. Front Nutr. 2023. PMID: 37964925 Free PMC article.
References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [Internet] [cited 2019 Jul 23] Available from: - DOI - PubMed
-
- Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1087172&tool=p... [Internet] [cited 2015 Feb 26] 1343–51. Available from. - PMC - PubMed
-
- Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146 http://www.ncbi.nlm.nih.gov/pubmed/24316260 [Internet] [cited 2015 Feb 10] 726–35. Available from. - PMC - PubMed
-
- Lee J.J., Lambert J.E., Hovhannisyan Y., Ramos-Roman M.A., Trombold J.R., Wagner D.A., et al. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101:34–43. http://www.ncbi.nlm.nih.gov/pubmed/25527748 [Internet] [cited 2019 Sep 4] Available from. - PMC - PubMed
-
- Che L., Chi W., Qiao Y., Zhang J., Song X., Liu Y., et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2019 http://gut.bmj.com/lookup/doi/10.1136/gutjnl-2018-317581 [Internet] [cited 2019 May 23];gutjnl-2018-317581. Available from: - DOI - PMC - PubMed
Grants and funding
- FS/15/56/31645/BHF_/British Heart Foundation/United Kingdom
- MR/P011462/1/MRC_/Medical Research Council/United Kingdom
- FS/SBSRF/21/31013/BHF_/British Heart Foundation/United Kingdom
- FS/19/18/34252/BHF_/British Heart Foundation/United Kingdom
- FS/14/17/30634/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources

