Acute intermittent hypoxia drives hepatic de novo lipogenesis in humans and rodents
- PMID: 35313531
- PMCID: PMC8933516
- DOI: 10.1016/j.metop.2022.100177
Acute intermittent hypoxia drives hepatic de novo lipogenesis in humans and rodents
Abstract
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver condition. It is tightly associated with an adverse metabolic phenotype (including obesity and type 2 diabetes) as well as with obstructive sleep apnoea (OSA) of which intermittent hypoxia is a critical component. Hepatic de novo lipogenesis (DNL) is a significant contributor to hepatic lipid content and the pathogenesis of NAFLD and has been proposed as a key pathway to target in the development of pharmacotherapies to treat NAFLD. Our aim is to use experimental models to investigate the impact of hypoxia on hepatic lipid metabolism independent of obesity and metabolic disease.
Methods: Human and rodent studies incorporating stable isotopes and hyperinsulinaemic euglycaemic clamp studies were performed to assess the regulation of DNL and broader metabolic phenotype by intermittent hypoxia. Cell-based studies, including pharmacological and genetic manipulation of hypoxia-inducible factors (HIF), were used to examine the underlying mechanisms.
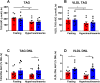
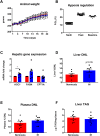
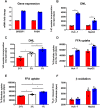
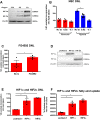
Results: Hepatic DNL increased in response to acute intermittent hypoxia in humans, without alteration in glucose production or disposal. These observations were endorsed in a prolonged model of intermittent hypoxia in rodents using stable isotopic assessment of lipid metabolism. Changes in DNL were paralleled by increases in hepatic gene expression of acetyl CoA carboxylase 1 and fatty acid synthase. In human hepatoma cell lines, hypoxia increased both DNL and fatty acid uptake through HIF-1α and -2α dependent mechanisms.
Conclusions: These studies provide robust evidence linking intermittent hypoxia and the regulation of DNL in both acute and sustained in vivo models of intermittent hypoxia, providing an important mechanistic link between hypoxia and NAFLD.
Keywords: HIF; Hypoxia; Lipid metabolism; NAFLD.
© 2022 The Authors.
Conflict of interest statement
None of the authors have any conflicts of interest or any relevant financial disclosures.
Figures

References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [Internet] [cited 2019 Jul 23] Available from: - DOI - PubMed
-
- Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1087172&tool=p... [Internet] [cited 2015 Feb 26] 1343–51. Available from. - PMC - PubMed
-
- Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146 http://www.ncbi.nlm.nih.gov/pubmed/24316260 [Internet] [cited 2015 Feb 10] 726–35. Available from. - PMC - PubMed
-
- Lee J.J., Lambert J.E., Hovhannisyan Y., Ramos-Roman M.A., Trombold J.R., Wagner D.A., et al. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101:34–43. http://www.ncbi.nlm.nih.gov/pubmed/25527748 [Internet] [cited 2019 Sep 4] Available from. - PMC - PubMed
-
- Che L., Chi W., Qiao Y., Zhang J., Song X., Liu Y., et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2019 http://gut.bmj.com/lookup/doi/10.1136/gutjnl-2018-317581 [Internet] [cited 2019 May 23];gutjnl-2018-317581. Available from: - DOI - PMC - PubMed
Grants and funding
- FS/15/56/31645/BHF_/British Heart Foundation/United Kingdom
- MR/P011462/1/MRC_/Medical Research Council/United Kingdom
- FS/SBSRF/21/31013/BHF_/British Heart Foundation/United Kingdom
- FS/19/18/34252/BHF_/British Heart Foundation/United Kingdom
- FS/14/17/30634/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources

