This is a preprint.
A new approach for extracting information from protein dynamics
- PMID: 35313540
- PMCID: PMC8936122
A new approach for extracting information from protein dynamics
Update in
-
A new approach for extracting information from protein dynamics.Proteins. 2023 Feb;91(2):183-195. doi: 10.1002/prot.26421. Epub 2022 Sep 29. Proteins. 2023. PMID: 36094321 Free PMC article.
Abstract
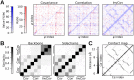
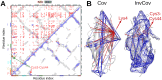
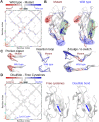
Increased ability to predict protein structures is moving research focus towards understanding protein dynamics. A promising approach is to represent protein dynamics through networks and take advantage of well-developed methods from network science. Most studies build protein dynamics networks from correlation measures, an approach that only works under very specific conditions, instead of the more robust inverse approach. Thus, we apply the inverse approach to the dynamics of protein dihedral angles, a system of internal coordinates, to avoid structural alignment. Using the well-characterized adhesion protein, FimH, we show that our method identifies networks that are physically interpretable, robust, and relevant to the allosteric pathway sites. We further use our approach to detect dynamical differences, despite structural similarity, for Siglec-8 in the immune system, and the SARS-CoV-2 spike protein. Our study demonstrates that using the inverse approach to extract a network from protein dynamics yields important biophysical insights.
Conflict of interest statement
Statements
Data available on request from the authors. We have no conflict of interest to declare.
Figures


Similar articles
-
A new approach for extracting information from protein dynamics.Proteins. 2023 Feb;91(2):183-195. doi: 10.1002/prot.26421. Epub 2022 Sep 29. Proteins. 2023. PMID: 36094321 Free PMC article.
-
Frustration-driven allosteric regulation and signal transmission in the SARS-CoV-2 spike omicron trimer structures: a crosstalk of the omicron mutation sites allosterically regulates tradeoffs of protein stability and conformational adaptability.Phys Chem Chem Phys. 2022 Jul 27;24(29):17723-17743. doi: 10.1039/d2cp01893d. Phys Chem Chem Phys. 2022. PMID: 35839100
-
Allosteric Control of Structural Mimicry and Mutational Escape in the SARS-CoV-2 Spike Protein Complexes with the ACE2 Decoys and Miniprotein Inhibitors: A Network-Based Approach for Mutational Profiling of Binding and Signaling.J Chem Inf Model. 2021 Oct 25;61(10):5172-5191. doi: 10.1021/acs.jcim.1c00766. Epub 2021 Sep 22. J Chem Inf Model. 2021. PMID: 34551245
-
In Silico Study of Allosteric Communication Networks in GPCR Signaling Bias.Int J Mol Sci. 2022 Jul 15;23(14):7809. doi: 10.3390/ijms23147809. Int J Mol Sci. 2022. PMID: 35887157 Free PMC article. Review.
-
Structural and Computational Studies of the SARS-CoV-2 Spike Protein Binding Mechanisms with Nanobodies: From Structure and Dynamics to Avidity-Driven Nanobody Engineering.Int J Mol Sci. 2022 Mar 8;23(6):2928. doi: 10.3390/ijms23062928. Int J Mol Sci. 2022. PMID: 35328351 Free PMC article. Review.
References
-
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., Bridgland A., Meyer C., Kohl S. A. A., Ballard A. J., Cowie A., Romera-Paredes B., Nikolov S., Jain R., Adler J., Back T., Petersen S., Reiman D., Clancy E., Zielinski M., Steinegger M., Pacholska M., Berghammer T., Bodenstein S., Silver D., Vinyals O., Senior A. W., Kavukcuoglu K., Kohli P., and Hassabis D., “Highly accurate protein structure prediction with AlphaFold,” Nature, pp. 1–11, 7 2021. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
