This is a preprint.
Omicron BA.1 and BA.2 neutralizing activity elicited by a comprehensive panel of human vaccines
- PMID: 35313570
- PMCID: PMC8936098
- DOI: 10.1101/2022.03.15.484542
Omicron BA.1 and BA.2 neutralizing activity elicited by a comprehensive panel of human vaccines
Update in
-
Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines.Science. 2022 Aug 19;377(6608):890-894. doi: 10.1126/science.abq0203. Epub 2022 Jul 19. Science. 2022. PMID: 35857529 Free PMC article.
Abstract
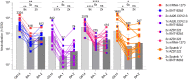
The SARS-CoV-2 Omicron variant of concern comprises three sublineages designated BA.1, BA.2, and BA.3, with BA.2 steadily replacing the globally dominant BA.1. We show that the large number of BA.1 and BA.2 spike mutations severely dampen plasma neutralizing activity elicited by infection or seven clinical vaccines, with cross-neutralization of BA.2 being consistently more potent than that of BA.1, independent of the vaccine platform and number of doses. Although mRNA vaccines induced the greatest magnitude of Omicron BA.1 and BA.2 plasma neutralizing activity, administration of a booster based on the Wuhan-Hu-1 spike sequence markedly increased neutralizing antibody titers and breadth against BA.1 and BA.2 across all vaccines evaluated. Our data suggest that although BA.1 and BA.2 evade polyclonal neutralizing antibody responses, current vaccine boosting regimens may provide sufficient protection against Omicron-induced disease.
Figures

References
-
- McCallum M., Bassi J., De Marco A., Chen A., Walls A. C., Di Iulio J., Tortorici M. A., Navarro M.-J., Silacci-Fregni C., Saliba C., Sprouse K. R., Agostini M., Pinto D., Culap K., Bianchi S., Jaconi S., Cameroni E., Bowen J. E., Tilles S. W., Pizzuto M. S., Guastalla S. B., Bona G., Pellanda A. F., Garzoni C., Van Voorhis W. C., Rosen L. E., Snell G., Telenti A., Virgin H. W., Piccoli L., Corti D., Veesler D., SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 373, 648–654 (2021). - PMC - PubMed
-
- Hodcroft E. B., Zuber M., Nadeau S., Vaughan T. G., Crawford K. H. D., Althaus C. L., Reichmuth M. L., Bowen J. E., Walls A. C., Corti D., Bloom J. D., Veesler D., Mateo D., Hernando A., Comas I., González-Candelas F., SeqCOVID-SPAIN consortium, Stadler T., Neher R. A., Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 595, 707–712 (2021). - PubMed
-
- McCallum M., Walls A. C., Sprouse K. R., Bowen J. E., Rosen L. E., Dang H. V., De Marco A., Franko N., Tilles S. W., Logue J., Miranda M. C., Ahlrichs M., Carter L., Snell G., Pizzuto M. S., Chu H. Y., Van Voorhis W. C., Corti D., Veesler D., Molecular basis of immune evasion by the Delta and Kappa SARS-CoV-2 variants. Science. 374, 1621–1626 (2021). - PubMed
-
- McCallum M., Czudnochowski N., Rosen L. E., Zepeda S. K., Bowen J. E., Walls A. C., Hauser K., Joshi A., Stewart C., Dillen J. R., Powell A. E., Croll T. I., Nix J., Virgin H. W., Corti D., Snell G., Veesler D., Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 375, 864–868 (2022). - PMC - PubMed
-
- Walls A. C., Sprouse K. R., Bowen J. E., Joshi A., Franko N., Navarro M. J., Stewart C., Cameroni E., McCallum M., Goecker E. A., Degli-Angeli E. J., Logue J., Greninger A., Corti D., Chu H. Y., Veesler D., SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell (2022), doi:10.1016/j.cell.2022.01.011. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
