Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis
- PMID: 35313615
- PMCID: PMC8926847
- DOI: 10.1016/j.lana.2022.100227
Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis
Abstract
Background: Real-world data on the effectiveness of boosters against COVID-19, especially as new variants continue to emerge, is limited. Our objective was to assess demographic, clinical, and outcome variables of patients requiring hospitalization for severe SARS-CoV-2 infection comparing fully vaccinated and boosted (FV&B), fully vaccinated (FV), and unvaccinated (UV) patients.
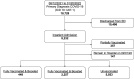
Methods: This multicenter observational cohort analysis compared demographic, clinical, and outcome variables in FV&B, FV, and UV adults hospitalized for COVID-19. Partially vaccinated (PV) and individuals still hospitalized beyond the designated follow-up date of February 1, 2022 were excluded. The primary endpoint was in-hospital mortality. Secondary endpoints included characteristics and outcomes in subpopulations of intensive care and geriatric (age >65) patients.
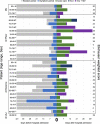
Findings: Between August 12th, 2021 and January 20th, 2022, 8232 patient encounters had a primary diagnosis of COVID-19 and required inpatient treatment. Of the 8232 encounters requiring hospitalization, 448 (5.8%) were FV&B, 2257 (29.2%) were FV, and 5023 (65.0%) were UV; 357 PV and 147 still hospitalized were excluded. The median age of FV&B cohort was 73 (IQR 62, 82) compared to 70 (IQR 59, 80) for FV and 59 (IQR 45, 71) for UV (0.001). Most patients were female in both the FB&V and UV groups with 51.1% and 51.8%, respectively, while the FV group had a majority of males (51.3%). The median Elixhauser weighted score was 12 (IQR 3, 22) for FV&B, 10 (IQR 2, 20) for FV, and 9 (IQR 0, 17) for UV groups (p < 0.001). In-hospital mortality was 7.1% in the FV&B, 10.3% in the FV group, and 12.8% in the UV group (p < 0.001). The FV&B group had lower in-hospital mortality than both FV and UV groups (p = 0.045 and p = 0.001, respectively). The FV group had lower in-hospital mortality than the UV group (p = 0.004).
Interpretation: Fully vaccinated and boosted patients requiring hospital-level care for breakthrough COVID-19 have lower in-hospital mortality than fully vaccinated and unvaccinated patients despite being older and higher risk at baseline. Boosters offer added protection beyond full vaccination in preventing death. As COVID-19 continues to spread, larger expansive trials are needed to further identify risk factors for severe outcomes among the FV&B population.
Funding: This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Keywords: Booster dose; COVID-19; Death; Hospitalization; Mortality; SARS-CoV-2; Severe illness; Vaccination.
© 2022 The Author(s).
Conflict of interest statement
All authors declare no relevant conflicts of interest relevant to this work.
Figures
References
-
- FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action...
-
- FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional...
-
- FDA issues emergency use authorization for third COVID-19 vaccine | FDA. Accessed June 18, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency...
-
- Provisional COVID-19 deaths by week, sex, and age | Data | Centers for Disease Control and Prevention. Accessed December 13, 2021. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-by-Week-Sex-and-Ag...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

