Effects of Dietary Chlorogenic Acid Supplementation Derived from Lonicera macranthoides Hand-Mazz on Growth Performance, Free Amino Acid Profile, and Muscle Protein Synthesis in a Finishing Pig Model
- PMID: 35313639
- PMCID: PMC8934221
- DOI: 10.1155/2022/6316611
Effects of Dietary Chlorogenic Acid Supplementation Derived from Lonicera macranthoides Hand-Mazz on Growth Performance, Free Amino Acid Profile, and Muscle Protein Synthesis in a Finishing Pig Model
Abstract
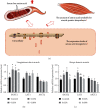
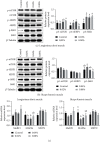
Chlorogenic acid (CGA), as one of the richest polyphenol compounds in nature, has broad applications in many fields due to its various biological properties. However, initial data on the effects of dietary CGA on protein synthesis and related basal metabolic activity has rarely been reported. The current study is aimed at (1) determining whether dietary CGA supplementation improves the growth performance and carcass traits, (2) assessing whether dietary CGA alters the free amino acid profile, and (3) verifying whether dietary CGA promotes muscle protein synthesis in finishing pigs. Thirty-two (Large × White × Landrace) finishing barrows with an average initial body weight of 71.89 ± 0.92 kg were randomly allotted to 4 groups and fed diets supplemented with 0, 0.02%, 0.04%, and 0.08% CGA, respectively. The results indicated that, compared with the control group, dietary supplementation with 0.04% CGA slightly stimulated the growth performance of pigs, whereas no significant correlation was noted between the dietary CGA levels and animal growth (P > 0.05). Furthermore, the carcass traits of pigs were improved by 0.04% dietary CGA (P < 0.01). In addition, dietary CGA significantly improved the serum free amino acid profiles of pigs (P < 0.01), while 0.04% dietary CGA promoted more amino acids to translocate to skeletal muscles (P < 0.05). The relative mRNA expression levels of SNAT2 in both longissimus dorsi (LD) and biceps femoris (BF) muscles were augmented in the 0.02% and 0.04% groups (P < 0.05), and the LAT1 mRNA expression in the BF muscle was elevated in the 0.02% group (P < 0.05). We also found that dietary CGA supplementation at the levels of 0.04% or 0.08% promoted the expression of p-Akt and activated the mTOR-S6K1-4EBP1 axis in the LD muscle (P < 0.05). Besides, the MAFbx mRNA abundance in the 0.02% and 0.04% groups was significantly lower (P < 0.05). Our results revealed that dietary supplementation with CGA of 0.04% improved the free amino acid profile and enhanced muscle protein biosynthesis in the LD muscle in finishing pigs.
Copyright © 2022 Wenlong Wang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Dietary Supplementation With Chlorogenic Acid Derived From Lonicera macranthoides Hand-Mazz Improves Meat Quality and Muscle Fiber Characteristics of Finishing Pigs via Enhancement of Antioxidant Capacity.Front Physiol. 2021 Apr 20;12:650084. doi: 10.3389/fphys.2021.650084. eCollection 2021. Front Physiol. 2021. PMID: 33959038 Free PMC article.
-
Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs.Food Funct. 2019 Nov 1;10(11):7426-7434. doi: 10.1039/c9fo01304k. Epub 2019 Oct 29. Food Funct. 2019. PMID: 31660546
-
Dietary supplementation of Lonicera macranthoides leaf powder improves amino acid profiles in serum and longissimus thoracis muscle of growing-finishing pigs.Anim Nutr. 2016 Dec;2(4):271-275. doi: 10.1016/j.aninu.2016.08.006. Epub 2016 Aug 21. Anim Nutr. 2016. PMID: 29767115 Free PMC article.
-
Bioactive functions of chlorogenic acid and its research progress in pig industry.J Anim Physiol Anim Nutr (Berl). 2024 Mar;108(2):439-450. doi: 10.1111/jpn.13905. Epub 2023 Nov 17. J Anim Physiol Anim Nutr (Berl). 2024. PMID: 37975278 Review.
-
Protein-Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications.Antioxidants (Basel). 2024 Jun 27;13(7):777. doi: 10.3390/antiox13070777. Antioxidants (Basel). 2024. PMID: 39061846 Free PMC article. Review.
Cited by
-
Effect of 3-caffeoylquinic acid on growth performance, nutrient digestibility, and intestinal functions in weaned pigs.J Anim Sci. 2023 Jan 3;101:skad234. doi: 10.1093/jas/skad234. J Anim Sci. 2023. PMID: 37422911 Free PMC article.
-
Magnolol Supplementation Alters Serum Parameters, Immune Homeostasis, Amino Acid Profiles, and Gene Expression of Amino Acid Transporters in Growing Pigs.Int J Mol Sci. 2023 Sep 11;24(18):13952. doi: 10.3390/ijms241813952. Int J Mol Sci. 2023. PMID: 37762256 Free PMC article.
-
Accounting Gut Microbiota as the Mediator of Beneficial Effects of Dietary (Poly)phenols on Skeletal Muscle in Aging.Nutrients. 2023 May 18;15(10):2367. doi: 10.3390/nu15102367. Nutrients. 2023. PMID: 37242251 Free PMC article. Review.
-
Effect of Different Levels of Chlorogenic Acid on Growth Performance, Immunological Responses, Antioxidant Defense, and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss) Juveniles.Aquac Nutr. 2023 Apr 19;2023:3679002. doi: 10.1155/2023/3679002. eCollection 2023. Aquac Nutr. 2023. PMID: 37124879 Free PMC article.
-
Ficus lindsayana Leaf Extract Protects C2C12 Mouse Myoblasts Against the Suppressive Effects of Bisphenol-A on Myogenic Differentiation.Int J Mol Sci. 2025 Jan 8;26(2):476. doi: 10.3390/ijms26020476. Int J Mol Sci. 2025. PMID: 39859191 Free PMC article.
References
-
- Lu H. J., Tian Z. M., Cui Y. Y., Liu Z. C., Ma X. Y. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Comprehensive Reviews in Food Science and Food Safety . 2020;19(6):3130–3158. doi: 10.1111/1541-4337.12620. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

