Cross-kingdom microbial interactions in dental implant-related infections: is Candida albicans a new villain?
- PMID: 35313695
- PMCID: PMC8933675
- DOI: 10.1016/j.isci.2022.103994
Cross-kingdom microbial interactions in dental implant-related infections: is Candida albicans a new villain?
Abstract
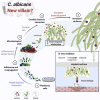
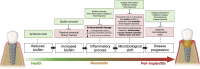
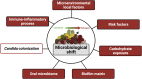
Candida albicans, an oral fungal opportunistic pathogen, has shown the ability to colonize implant surfaces and has been frequently isolated from biofilms associated with dental implant-related infections, possibly due to its synergistic interactions with certain oral bacteria. Moreover, evidence suggests that this cross-kingdom interaction on implant can encourage bacterial growth, leading to increased fungal virulence and mucosal damage. However, the role of Candida in implant-related infections has been overlooked and not widely explored or even considered by most microbiological analyses and therapeutic approaches. Thus, we summarized the scientific evidence regarding the ability of C. albicans to colonize implant surfaces, interact in implant-related polymicrobial biofilms, and its possible role in peri-implant infections as far as biologic plausibility. Next, a systematic review of preclinical and clinical studies was conducted to identify the relevance and the gap in the existing literature regarding the role of C. albicans in the pathogenesis of peri-implant infections.
Keywords: Mycology; microbiofilms.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Biofilm Interactions of Candida albicans and Mitis Group Streptococci in a Titanium-Mucosal Interface Model.Appl Environ Microbiol. 2020 Apr 17;86(9):e02950-19. doi: 10.1128/AEM.02950-19. Print 2020 Apr 17. Appl Environ Microbiol. 2020. PMID: 32111586 Free PMC article.
-
Cross-kingdom interaction between Candida albicans and oral bacteria.Front Microbiol. 2022 Nov 3;13:911623. doi: 10.3389/fmicb.2022.911623. eCollection 2022. Front Microbiol. 2022. PMID: 36406433 Free PMC article. Review.
-
Candida-Bacterial Biofilms and Host-Microbe Interactions in Oral Diseases.Adv Exp Med Biol. 2019;1197:119-141. doi: 10.1007/978-3-030-28524-1_10. Adv Exp Med Biol. 2019. PMID: 31732939 Review.
-
In it together: Candida-bacterial oral biofilms and therapeutic strategies.Environ Microbiol Rep. 2022 Apr;14(2):183-196. doi: 10.1111/1758-2229.13053. Epub 2022 Feb 26. Environ Microbiol Rep. 2022. PMID: 35218311 Free PMC article. Review.
-
Contributions of Candida albicans Dimorphism, Adhesive Interactions, and Extracellular Matrix to the Formation of Dual-Species Biofilms with Streptococcus gordonii.mBio. 2019 Jun 18;10(3):e01179-19. doi: 10.1128/mBio.01179-19. mBio. 2019. PMID: 31213561 Free PMC article.
Cited by
-
Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases.Microorganisms. 2022 Dec 6;10(12):2413. doi: 10.3390/microorganisms10122413. Microorganisms. 2022. PMID: 36557666 Free PMC article.
-
Subgingival Yeasts Species Amongst Smokers and Nonsmokers With Peri-implantitis.Int Dent J. 2025 Apr;75(2):960-969. doi: 10.1016/j.identj.2024.09.029. Epub 2024 Oct 23. Int Dent J. 2025. PMID: 39443261 Free PMC article.
-
Oral Mycobiota: A Narrative Review.Dent J (Basel). 2024 Apr 19;12(4):115. doi: 10.3390/dj12040115. Dent J (Basel). 2024. PMID: 38668027 Free PMC article. Review.
-
Controlling Oral Polymicrobial Biofilm Using Usnic Acid on the Surface of Titanium in the Artificial Saliva Media.Antibiotics (Basel). 2025 Jan 22;14(2):115. doi: 10.3390/antibiotics14020115. Antibiotics (Basel). 2025. PMID: 40001359 Free PMC article.
-
The effect of dental material type and masticatory forces on periodontitis-derived subgingival microbiomes.Biofilm. 2024 May 8;7:100199. doi: 10.1016/j.bioflm.2024.100199. eCollection 2024 Jun. Biofilm. 2024. PMID: 38800100 Free PMC article.
References
-
- Ahmed A., Chambers M.S., Goldschmidt M.C., Habib A., Lei X., Jacob R.F. Association between microbial flora and tissue abnormality around dental implants penetrating the skin in reconstructed oral cancer patients. Int. J. Oral Maxill. Implants. 2012;27:684–694. - PubMed
-
- Alsahhaf A., Al-Aali K.A., Alshagroud R.S., Alshiddi I.F., Alrahlah A., Abduljabbar T., Javed F., Vohra F. Comparison of yeast species in the subgingival oral biofilm of individuals with type 2 diabetes and peri-implantitis and individuals with peri-implantitis without diabetes. J. Periodontol. 2019;90:1383–1389. doi: 10.1002/JPER.19-0091. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

