Discontinuation rates of intrauterine contraception due to unfavourable bleeding: a systematic review
- PMID: 35313863
- PMCID: PMC8939098
- DOI: 10.1186/s12905-022-01657-6
Discontinuation rates of intrauterine contraception due to unfavourable bleeding: a systematic review
Abstract
Objective: Levonorgestrel-releasing intrauterine devices (LNG-IUDs) and copper intrauterine devices (Cu-IUDs) offer long-acting contraception; however, some women may discontinue use within the first year due to bleeding pattern changes, limiting their potential. This systematic literature review investigated whether differences in bleeding profiles influence continuation rates in women in America, Europe and Australia.
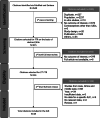
Methods: Searches performed in PubMed and Embase were screened to identify publications describing bleeding patterns and rates of early IUC removal/discontinuation or continuation, descriptions of bleeding patterns, reasons for discontinuation, and patient satisfaction, acceptability and tolerability for LNG-IUDs and Cu-IUDs published between January 2010 and December 2019. The results were further restricted to capture citations related to 'Humans' and 'Females'. The review was limited to studies published from 2010 onwards, as changing attitudes over time mean that results of studies performed before this date may not be generalizable to current practice.
Results: Forty-eight publications describing 41 studies performed principally in the USA (n = 17) and Europe (n = 13) were identified. Publications describing bleeding patterns in LNG-IUD users (n = 11) consistently observed a reduction in bleeding in most women, whereas two of three studies in Cu-IUD users reported heavy bleeding in approximately 40% of patients. Rates of discontinuation for both devices ranged widely and may be as high as 50% but were lower for LNG-IUDs versus Cu-IUDs. Discontinuation rates due to bleeding were consistently higher for Cu-IUDs versus LNG-IUDs.
Conclusions: Bleeding is a common reason for discontinuation of Cu-IUDs and LNG-IUDs. The more favourable bleeding pattern observed in LNG-IUD users may be associated with a lower rate of early discontinuation of LNG-IUDs versus Cu-IUDs.
Keywords: Contraception; Discontinuation; Intrauterine device; Menstrual bleeding; Satisfaction.
© 2022. The Author(s).
Conflict of interest statement
DC has received grants and personal fees from Bayer AG, Merck and Allergan. RC is an employee of Accuscript Consultancy, Ludhiana, Punjab, India. RH is an employee of Accuscript Consultancy, Reading, Berkshire, UK. ST has served on scientific advisory boards for Allergan and Bayer AG. MM is an employee of Bayer AG, Berlin, Germany. Accuscript Consultancy was paid a fee for the conduct of the initial literature search and analysis.
Figures


References
-
- Blumenthal PD, Voedisch A, Gemzell-Danielsson K. Strategies to prevent unintended pregnancy: increasing use of long-acting reversible contraception. Hum Reprod Update. 2011;17(1):121–137. - PubMed
-
- U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1–60. - PubMed
-
- Ortiz ME, Croxatto HB. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception. 2007;75(6 Suppl):S16–30. - PubMed
-
- Bayer HealthCare Pharmaceuticals Inc. Mirena Prescribing Information. October 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021225s027lbl.pdf. Accessed Jan 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

