Mirabegron for overactive bladder in frail patients 80 years or over (HOKUTO study)
- PMID: 35313873
- PMCID: PMC8939141
- DOI: 10.1186/s12894-022-00989-7
Mirabegron for overactive bladder in frail patients 80 years or over (HOKUTO study)
Abstract
Background: We assessed the efficacy and safety of mirabegron, a β3-adrenoceptor agonist, in older adults (≥ 80 years old) with overactive bladder (OAB).
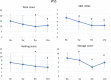
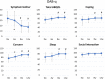
Methods: OAB patients aged ≥ 80 years were enrolled in this prospective, single-arm observational study. OAB was diagnosed based on the OAB symptom score (OABSS); i.e., a total score of ≥ 3 points and an urgency score of ≥ 2 points. Patients who received 50 mg mirabegron once daily were evaluated at the baseline and at 4, 8, and 12 weeks. The changes from the baseline in the OABSS, International Prostate Symptom Score (IPSS), OAB questionnaire (OAB-q) score, and Vulnerable Elders Survey (VES-13) score were determined. Adverse events, laboratory tests, 12-lead electrocardiography, the QT interval according to Fridericia's formula (QTcF), uroflowmetry, the post-void residual urine volume (PVR), and the Mini-Mental State Examination (MMSE) score were used to assess safety.
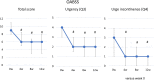
Results: Forty-three patients (median age: 84 years, range: 80-96 years) were examined. They had high rates of comorbidities and polypharmacy. Mirabegron significantly improved in total score of the OABSS, including urgency and urge incontinence. The total IPSS, IPSS quality-of-life (QOL) index, and OAB-q scores also significantly improved. Mirabegron improved in the VES-13 score. There were no significant changes in laboratory test values, uroflowmetry findings, PVR, the QTcF, or MMSE score. Two patients (4.7%) withdrew from the study after experiencing adverse events.
Conclusions: Mirabegron was well tolerated and significantly improved in OAB symptoms, and QOL in older patients. Trial registration The present clinical study was approved by University of Yamanashi Institutional Review Board prior to study initiation (ID1447) and was retrospectively registered with the UMIN Clinical Trials Registry (UMIN-CTR), Japan (UMIN000045996) on Nov 6, 2021.
Keywords: Frail; OAB; Older; Older society; β3-adrenoceptor agonist.
© 2022. The Author(s).
Conflict of interest statement
There are no potential competing interests to declare.
Figures
Comment in
-
Geriatrics.J Urol. 2022 Oct;208(4):909-911. doi: 10.1097/JU.0000000000002876. Epub 2022 Jul 28. J Urol. 2022. PMID: 35900810 No abstract available.
References
-
- Yamagami H, Imura M, Hiro S. The demographics and treatment circumstances of patients receiving OAB treatment medication. Jpn J Urol Surg. 2014;27:1183–1190.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials