Exploring ITM2A as a new potential target for brain delivery
- PMID: 35313913
- PMCID: PMC8935840
- DOI: 10.1186/s12987-022-00321-3
Exploring ITM2A as a new potential target for brain delivery
Abstract
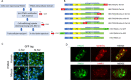
Background: Integral membrane protein 2A (ITM2A) is a transmembrane protein expressed in a variety of tissues; little is known about its function, particularly in the brain. ITM2A was found to be highly enriched in human brain versus peripheral endothelial cells by transcriptomic and proteomic studies conducted within the European Collaboration on the Optimization of Macromolecular Pharmaceutical (COMPACT) Innovative Medicines Initiative (IMI) consortium. Here, we report the work that was undertaken to determine whether ITM2A could represent a potential target for delivering drugs to the brain.
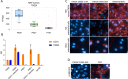
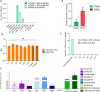
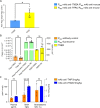
Methods: A series of ITM2A constructs, cell lines and specific anti-human and mouse ITM2A antibodies were generated. Binding and internalization studies in Human Embryonic Kidney 293 (HEK293) cells overexpressing ITM2A and in brain microvascular endothelial cells from mouse and non-human primate (NHP) were performed with these tools. The best ITM2A antibody was evaluated in an in vitro human blood brain barrier (BBB) model and in an in vivo mouse pharmacokinetic study to investigate its ability to cross the BBB.
Results: Antibodies specifically recognizing extracellular parts of ITM2A or tags inserted in its extracellular domain showed selective binding and uptake in ITM2A-overexpressing cells. However, despite high RNA expression in mouse and human microvessels, the ITM2A protein was rapidly downregulated when endothelial cells were grown in culture, probably explaining why transcytosis could not be observed in vitro. An attempt to directly demonstrate in vivo transcytosis in mice was inconclusive, using either a cross-reactive anti-ITM2A antibody or in vivo phage panning of an anti-ITM2A phage library.
Conclusions: The present work describes our efforts to explore the potential of ITM2A as a target mediating transcytosis through the BBB, and highlights the multiple challenges linked to the identification of new brain delivery targets. Our data provide evidence that antibodies against ITM2A are internalized in ITM2A-overexpressing HEK293 cells, and that ITM2A is expressed in brain microvessels, but further investigations will be needed to demonstrate that ITM2A is a potential target for brain delivery.
Keywords: Antibodies; Blood brain barrier; ITM2A; Transcytosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Targeting blood-brain-barrier transcytosis - perspectives for drug delivery.Neuropharmacology. 2017 Jul 1;120:4-7. doi: 10.1016/j.neuropharm.2016.08.025. Epub 2016 Aug 22. Neuropharmacology. 2017. PMID: 27561970 Review.
-
Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies.Neuron. 2016 Jan 6;89(1):70-82. doi: 10.1016/j.neuron.2015.11.024. Epub 2015 Dec 10. Neuron. 2016. PMID: 26687840
-
Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity.J Neurochem. 2018 Sep;146(6):735-752. doi: 10.1111/jnc.14482. Epub 2018 Aug 16. J Neurochem. 2018. PMID: 29877588 Free PMC article.
-
Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human.Fluids Barriers CNS. 2020 Jul 22;17(1):47. doi: 10.1186/s12987-020-00209-0. Fluids Barriers CNS. 2020. PMID: 32698806 Free PMC article.
-
Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors.Expert Opin Drug Deliv. 2019 Jun;16(6):583-605. doi: 10.1080/17425247.2019.1614911. Epub 2019 May 20. Expert Opin Drug Deliv. 2019. PMID: 31107110 Review.
Cited by
-
Acute severe hypoglycemia alters mouse brain microvascular proteome.J Cereb Blood Flow Metab. 2024 Apr;44(4):556-572. doi: 10.1177/0271678X231212961. Epub 2023 Nov 9. J Cereb Blood Flow Metab. 2024. PMID: 37944245 Free PMC article.
-
Direct interoceptive input to the insular cortex shapes learned feeding behavior.bioRxiv [Preprint]. 2025 May 17:2025.05.13.653896. doi: 10.1101/2025.05.13.653896. bioRxiv. 2025. PMID: 40462995 Free PMC article. Preprint.
-
An easy-to-perform method for microvessel isolation and primary brain endothelial cell culture to study Alzheimer's disease.Heliyon. 2024 Jun 15;10(12):e33077. doi: 10.1016/j.heliyon.2024.e33077. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38994107 Free PMC article.
-
Identification of Cell Fate Determining Transcription Factors for Generating Brain Endothelial Cells.Stem Cell Rev Rep. 2025 Apr;21(3):744-766. doi: 10.1007/s12015-025-10842-7. Epub 2025 Jan 24. Stem Cell Rev Rep. 2025. PMID: 39853537 Free PMC article.
-
Exploring the Role of Inflammation and Metabolites in Bell's Palsy and Potential Treatment Strategies.Biomedicines. 2025 Apr 13;13(4):957. doi: 10.3390/biomedicines13040957. Biomedicines. 2025. PMID: 40299566 Free PMC article.
References
-
- Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. - PubMed
-
- Lesuisse D. Increasing brain exposure of antibodies. In: de Lange ECM, Hammarlund-Udenaes M, Thorne RG, editors. Drug delivery to the brain. 2. New York: Springer; 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

