The innate immune receptor Nlrp12 suppresses autoimmunity to the retina
- PMID: 35313917
- PMCID: PMC8939070
- DOI: 10.1186/s12974-022-02425-x
The innate immune receptor Nlrp12 suppresses autoimmunity to the retina
Abstract
Background: Nod-like receptors (NLRs) are critical to innate immune activation and induction of adaptive T cell responses. Yet, their role in autoinflammatory diseases of the central nervous system (CNS) remains incompletely defined. The NLR, Nlrp12, has been reported to both inhibit and promote neuroinflammation in an animal model of multiple sclerosis (experimental autoimmune encephalomyelitis, EAE), where its T cell-specific role has been investigated. Uveitis resulting from autoimmunity of the neuroretina, an extension of the CNS, involves a breach in immune privilege and entry of T cells into the eye. Here, we examined the contribution of Nlrp12 in a T cell-mediated model of uveitis, experimental autoimmune uveitis (EAU).
Methods: Mice were immunized with interphotoreceptor retinoid-binding protein peptide 1-20 (IRBP1-20) emulsified in Complete Freund's adjuvant, CFA. Uveitis was evaluated by clinical and histopathological scoring, and comparisons were made in WT vs. Nlrp12-/- mice, lymphopenic Rag1-/- mice reconstituted with WT vs. Nlrp12-/- CD4+ T cells, or among bone marrow (BM) chimeric mice. Antigen-specific Th-effector responses were evaluated by ELISA and intracellular cytokine staining. Cellular composition of uveitic eyes from WT or Nlrp12-/- mice was compared using flow cytometry. Expression of Nlrp12 and of cytokines/chemokines within the neuroretina was evaluated by immunoblotting and quantitative PCR.
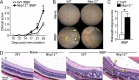
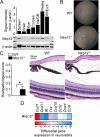
Results: Nlrp12-/- mice developed exacerbated uveitis characterized by extensive vasculitis, chorioretinal infiltrates and photoreceptor damage. Nlrp12 was dispensable for T cell priming and differentiation of peripheral Th1 or Th17 cells, and uveitis in immunodeficient mice reconstituted with either Nlrp12-/- or WT T cells was similar. Collectively, this ruled out T cells as the source of Nlrp12-mediated protection to EAU. Uveitic Nlrp12-/- eyes had more pronounced myeloid cell accumulation than uveitic WT eyes. Transplantation of Nlrp12-/- BM resulted in increased susceptibility to EAU regardless of host genotype, but interestingly, a non-hematopoietic origin for Nlrp12 function was also observed. Indeed, Nlrp12 was found to be constitutively expressed in the neuroretina, where it suppressed chemokine/cytokine induction.
Conclusions: Our data identify a combinatorial role for Nlrp12 in dampening autoimmunity of the neuroretina. These findings could provide a pathway for development of therapies for uveitis and potentially other autoinflammatory/autoimmune diseases of the CNS.
Keywords: Nlrp12; Nod-like receptor; Pattern recognition receptor; Uveitis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen.J Autoimmun. 2013 Aug;44:21-33. doi: 10.1016/j.jaut.2013.06.003. Epub 2013 Jun 28. J Autoimmun. 2013. PMID: 23810578 Free PMC article.
-
The nod-like receptor, Nlrp12, plays an anti-inflammatory role in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2015 Oct 31;12:198. doi: 10.1186/s12974-015-0414-5. J Neuroinflammation. 2015. PMID: 26521018 Free PMC article.
-
Mincle Activation and the Syk/Card9 Signaling Axis Are Central to the Development of Autoimmune Disease of the Eye.J Immunol. 2016 Apr 1;196(7):3148-58. doi: 10.4049/jimmunol.1502355. Epub 2016 Feb 26. J Immunol. 2016. PMID: 26921309 Free PMC article.
-
The Role of Adenosine in γδ T-Cell Regulation of Th17 Responses in Experimental Autoimmune Uveitis.Biomolecules. 2023 Sep 22;13(10):1432. doi: 10.3390/biom13101432. Biomolecules. 2023. PMID: 37892114 Free PMC article. Review.
-
Microbiome and Autoimmune Uveitis.Front Immunol. 2019 Feb 19;10:232. doi: 10.3389/fimmu.2019.00232. eCollection 2019. Front Immunol. 2019. PMID: 30837991 Free PMC article. Review.
Cited by
-
Spotlight on pyroptosis: role in pathogenesis and therapeutic potential of ocular diseases.J Neuroinflammation. 2022 Jul 14;19(1):183. doi: 10.1186/s12974-022-02547-2. J Neuroinflammation. 2022. PMID: 35836195 Free PMC article. Review.
References
-
- Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10(4):263–279. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

