Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized, multiple-blind, placebo-controlled trial
- PMID: 35313933
- PMCID: PMC8935852
- DOI: 10.1186/s13063-022-06142-x
Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized, multiple-blind, placebo-controlled trial
Abstract
Introduction: Diarrhea-predominant irritable bowel syndrome (IBS-D) is a bowel disease with a high incidence. It significantly reduces the quality of life of patients and affects the patient's daily activities and mental health. No specific therapeutic medications for IBS-D have been found. Published clinical trials suggest that Chinese herbal formula Tongxie Yaofang (TXYF) for IBS-D may be effective. However, high-quality clinical evidence supporting its use in IBS-D is still lacking. This trial aims to evaluate the therapeutic effects and safety of TXYF for IBS-D in adults.
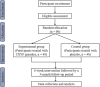
Methods/design: A randomized, multiple-blind, placebo-controlled trial will be conducted. It will consist of an 8-week intervention followed by a 3-month follow-up period. The target sample size is 96 IBS-D patients aged 18 to 65 years. The eligible participants will be randomly allocated to either TXYF or placebo group in a ratio of 1:1. Participants in the experimental group will take TXYF granules, while participants in the control group will be given TXYF placebo granules. The primary outcome will be the degree of IBS symptom severity measured using the scale of IBS symptom severity score. The secondary outcomes include: (a) stool frequency, and (b) stool consistency measured using the Bristol stool scale, (c) quality of life measured using the scale of IBS-quality of life, (d) anxiety measured using the self-rating anxiety scale, (e) depression measured by the self-rating depression scale, and (f) the safety of using TXYF and placebo. Safety monitoring and assessment will be undertaken throughout treatment.
Discussion: Chinese herbal formula TXYF consists of four Chinese herbs: Atractylodes macrocephala Koidz., Paeonia lactiflora Pall ., Citrus × aurantium L ., and Radix saposhnikoviae. It has been used for diarrhea for hundreds of years and may have a potential benefit in treating adults with IBS-D, but due to lack of high-quality evidence, we designed a randomized, multiple-blind, placebo-controlled trial to evaluate its therapeutic effects and safety. This trial will provide important data to guide the clinical practice of TXYF for the treatment of IBS-D in adults.
Trial registration: ISRCTN registry ISRCTN12453166 . Registered on 23 March 2021.
Keywords: Chinese herbal medicine; IBS-D; Irritable bowel syndrome; Randomized controlled trial; Tongxie Yaofang.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Systematic review and meta-analysis of Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: Evidence for clinical practice and future trials.Front Pharmacol. 2022 Aug 25;13:904657. doi: 10.3389/fphar.2022.904657. eCollection 2022. Front Pharmacol. 2022. PMID: 36091782 Free PMC article.
-
Chinese herbal formula Tongxie Yaofang granules for diarrhoea-predominant irritable bowel syndrome: a randomised, double-blind, placebo-controlled, phase II trial.BMJ Open. 2025 Jan 27;15(1):e088410. doi: 10.1136/bmjopen-2024-088410. BMJ Open. 2025. PMID: 39870499 Free PMC article. Clinical Trial.
-
Efficacy of Tong-Xie-Yao-Fang granule and its impact on whole transcriptome profiling in diarrhea-predominant irritable bowel syndrome patients: study protocol for a randomized controlled trial.Trials. 2020 Nov 3;21(1):908. doi: 10.1186/s13063-020-04833-x. Trials. 2020. PMID: 33143731 Free PMC article.
-
How Tongxie-Yaofang Regulates Intestinal Synaptic Plasticity by Activating Enteric Glial Cells and NGF/TrkA Pathway in Diarrhea-Predominant Irritable Bowel Syndrome Rats.Drug Des Devel Ther. 2023 Sep 27;17:2969-2983. doi: 10.2147/DDDT.S423333. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789966 Free PMC article.
-
Different therapies of Chinese herbal medicine for diarrhea-predominant irritable bowel syndrome: A network meta-analysis of double-blinded, placebo-controlled trials.J Ethnopharmacol. 2023 Dec 5;317:116672. doi: 10.1016/j.jep.2023.116672. Epub 2023 Jun 14. J Ethnopharmacol. 2023. PMID: 37328079 Review.
Cited by
-
Systematic review and meta-analysis of Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: Evidence for clinical practice and future trials.Front Pharmacol. 2022 Aug 25;13:904657. doi: 10.3389/fphar.2022.904657. eCollection 2022. Front Pharmacol. 2022. PMID: 36091782 Free PMC article.
-
Exploring the synergistic pharmacological mechanism of Huoxiang Drink against irritable bowel syndrome by integrated data mining and network pharmacology.Medicine (Baltimore). 2023 Sep 29;102(39):e35220. doi: 10.1097/MD.0000000000035220. Medicine (Baltimore). 2023. PMID: 37773835 Free PMC article.
-
Chinese herbal medicine, Tongxieyaofang, alleviates diarrhea via gut microbiota remodeling: evidence from network pharmacology and full-length 16S rRNA gene sequencing.Front Cell Infect Microbiol. 2024 Nov 15;14:1502373. doi: 10.3389/fcimb.2024.1502373. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39619663 Free PMC article.
-
Chinese herbal formula Tongxie Yaofang granules for diarrhoea-predominant irritable bowel syndrome: a randomised, double-blind, placebo-controlled, phase II trial.BMJ Open. 2025 Jan 27;15(1):e088410. doi: 10.1136/bmjopen-2024-088410. BMJ Open. 2025. PMID: 39870499 Free PMC article. Clinical Trial.
-
Atractylenolide I ameliorates post-infectious irritable bowel syndrome by inhibiting the polymerase I and transcript release factor and c-Jun N-terminal kinase/inducible nitric oxide synthase pathway.J Tradit Chin Med. 2025 Feb;45(1):57-65. doi: 10.19852/j.cnki.jtcm.2025.01.006. J Tradit Chin Med. 2025. PMID: 39957159 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources