Development of two species of the Trypanosoma theileri complex in tabanids
- PMID: 35313955
- PMCID: PMC8935851
- DOI: 10.1186/s13071-022-05212-y
Development of two species of the Trypanosoma theileri complex in tabanids
Abstract
Background: Trypanosoma theileri species complex includes parasites of Bovidae (cattle, sheep, goat, etc.) and Cervidae (deer) transmitted mainly by Tabanidae (horse flies and deerflies) and keds (Hippoboscidae). While morphological discrimination of species is challenging, two big clades, TthI and TthII, each containing parasites isolated from bovids and cervids, have been identified phylogenetically. To date, the development in the vector has been studied in detail only for the ked-transmitted sheep parasite T. melophagium (TthII), while the fate of trypanosomes in tabanids was described only briefly by light microscopy.
Methods: We collected infected tabanids of various species and identified trypanosomes by molecular phylogenetic analysis. The morphology and development of trypanosomes was studied using the combination of statistical analyses as well as light and electron microscopy.
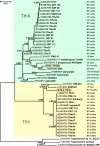
Results: Two trypanosome species belonging to both TthI and TthII clades of the T. theileri complex were identified. The phylogenetic position of these two trypanosomes suggests that they parasitize deer. Both species were indiscernible by morphology in the vector and showed the same development in its intestine. In contrast to the previously described development of T. melophagium, both trypanosomes of tabanids only transiently infected midgut and settled mainly in the ileum, while pylorus and rectum were neglected. Meanwhile, the flagellates developing in the tabanid ileum (pyriform epimastigotes and metacyclic trypomastigotes) showed similarities to the corresponding stages in T. melophagium by morphology, mode of attachment to the host cuticle and formation of the fibrillar matrix surrounding the mass of developing parasites. In addition, for the first time to our knowledge we documented extraintestinal stages in these trypanosomes, located in the space between the epithelium and circular muscles.
Conclusions: The development of different species of flagellates of the T. theileri complex in their insect vectors shows many similarities, which can be explained not only by their common origin, but also the same transmission mode, i.e. contamination of the oral mucosa with the gut content released after squashing the insect either by tongue or teeth. The observed differences (concerning primarily the distribution of developmental stages in the intestine) are associated rather with the identity of vectors than the phylogenetic position of parasites.
Keywords: Deerflies; Horseflies; Life cycle; Trypanosomes; Vector.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









Similar articles
-
Pan-American Trypanosoma (Megatrypanum) trinaperronei n. sp. in the white-tailed deer Odocoileus virginianus Zimmermann and its deer ked Lipoptena mazamae Rondani, 1878: morphological, developmental and phylogeographical characterisation.Parasit Vectors. 2020 Jun 12;13(1):308. doi: 10.1186/s13071-020-04169-0. Parasit Vectors. 2020. PMID: 32532317 Free PMC article.
-
Trypanosoma (Megatrypanum) melophagium in the sheep ked Melophagus ovinus from organic farms in Croatia: phylogenetic inferences support restriction to sheep and sheep keds and close relationship with trypanosomes from other ruminant species.J Eukaryot Microbiol. 2012 Mar-Apr;59(2):134-44. doi: 10.1111/j.1550-7408.2011.00599.x. Epub 2011 Dec 13. J Eukaryot Microbiol. 2012. PMID: 22168919
-
Trypanosomes of the Trypanosoma theileri Group: Phylogeny and New Potential Vectors.Microorganisms. 2022 Jan 26;10(2):294. doi: 10.3390/microorganisms10020294. Microorganisms. 2022. PMID: 35208749 Free PMC article.
-
Trypanosomes of Australian mammals: A review.Int J Parasitol Parasites Wildl. 2014 Mar 15;3(2):57-66. doi: 10.1016/j.ijppaw.2014.02.002. eCollection 2014 Aug. Int J Parasitol Parasites Wildl. 2014. PMID: 25161902 Free PMC article. Review.
-
Tabanids: neglected subjects of research, but important vectors of disease agents!Infect Genet Evol. 2014 Dec;28:596-615. doi: 10.1016/j.meegid.2014.03.029. Epub 2014 Apr 13. Infect Genet Evol. 2014. PMID: 24727644 Review.
Cited by
-
Genetic Diversity of Trypanosomes Infesting Cattle from Savannah District in North of Côte d'Ivoire Using Conserved Genomic Signatures: rRNA, ITS1 and gGAPDH.Pathogens. 2024 Mar 19;13(3):262. doi: 10.3390/pathogens13030262. Pathogens. 2024. PMID: 38535605 Free PMC article.
-
Parasites of firebugs in Austria with focus on the "micro"-diversity of the cosmopolitan trypanosomatid Leptomonas pyrrhocoris.Parasitol Res. 2023 Dec 11;123(1):27. doi: 10.1007/s00436-023-08080-2. Parasitol Res. 2023. PMID: 38072883 Free PMC article.
-
Seasonal Variation and Factors Affecting Trypanosoma theileri Infection in Wild Sika Deer (Ezo Sika Deer Cervus nippon yesoensis) in Eastern Hokkaido.Animals (Basel). 2023 May 22;13(10):1707. doi: 10.3390/ani13101707. Animals (Basel). 2023. PMID: 37238137 Free PMC article.
-
Molecular Detection and Analysis of Trypanosoma (Megatrypanum) spp. Diversity in Tabanidae (Diptera) Collected in Lithuania.Insects. 2024 Jul 30;15(8):581. doi: 10.3390/insects15080581. Insects. 2024. PMID: 39194786 Free PMC article.
-
First Record of Leishmania (Viannia) sp. and High Prevalence of Anaplasma marginale and Trypanosoma theileri in Zebu Cattle from Zenú Communities in Northern Colombia.Pathogens. 2025 Apr 15;14(4):382. doi: 10.3390/pathogens14040382. Pathogens. 2025. PMID: 40333177 Free PMC article.
References
-
- Podlipaev SA. Catalogue of world fauna of Trypanosomatidae (Protozoa) Leningrad: Zoologicheskii Institut AN SSSR; 1990.
-
- Hoare CA. The trypanosomes of mammals. A zoological monograph. Oxford: Blackwell Scientific Publications; 1972.
-
- Bruce D, Hamerton AE, Bateman HR, Mackie FP. Trypanosomaingens, n. sp. Proc R Soc Lond. 1909;81:323–324.
-
- Kingston N, Morton JK. Trypanosoma cervi sp. n. from elk (Cervuscanadensis) in Wyoming. J Parasitol. 1975;61:17–23. - PubMed
-
- Kingston N, Bobek B, Perzanowski K, Wita I, Maki L. Description of Trypanosoma (Megatrypanum) stefanskii sp. N. from roe deer (Capreoluscapreolus) in Poland. J Helminthol Soc Wash. 1992;59:89–95.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

