Thyroid hormone enhances stem cell maintenance and promotes lineage-specific differentiation in human embryonic stem cells
- PMID: 35313973
- PMCID: PMC8935725
- DOI: 10.1186/s13287-022-02799-y
Thyroid hormone enhances stem cell maintenance and promotes lineage-specific differentiation in human embryonic stem cells
Abstract
Background: Thyroid hormone triiodothyronine (T3) is essential for embryogenesis and is commonly used during in vitro fertilization to ensure successful implantation. However, the regulatory mechanisms of T3 during early embryogenesis are largely unknown.
Method: To study the impact of T3 on hPSCs, cell survival and growth were evaluated by measurement of cell growth curve, cloning efficiency, survival after passaging, cell apoptosis, and cell cycle status. Pluripotency was evaluated by RT-qPCR, immunostaining and FACS analysis of pluripotency markers. Metabolic status was analyzed using LC-MS/MS and Seahorse XF Cell Mito Stress Test. Global gene expression was analyzed using RNA-seq. To study the impact of T3 on lineage-specific differentiation, cells were subjected to T3 treatment during differentiation, and the outcome was evaluated using RT-qPCR, immunostaining and FACS analysis of lineage-specific markers.
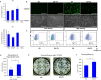
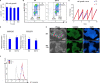
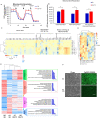
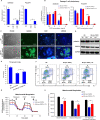
Results: In this report, we use human pluripotent stem cells (hPSCs) to show that T3 is beneficial for stem cell maintenance and promotes trophoblast differentiation. T3 enhances culture consistency by improving cell survival and passaging efficiency. It also modulates cellular metabolism and promotes energy production through oxidative phosphorylation. T3 helps maintain pluripotency by promoting ERK and SMAD2 signaling and reduces FGF2 dependence in chemically defined culture. Under BMP4 induction, T3 significantly enhances trophoblast differentiation.
Conclusion: In summary, our study reveals the impact of T3 on stem cell culture through signal transduction and metabolism and highlights its potential role in improving stem cell applications.
Keywords: Cell culture; Pluripotency; Thyroid Hormone; Triiodothyronine (T3); Trophoblast; hPSCs.
© 2022. The Author(s).
Conflict of interest statement
C.D. and G.C. filed a patent application based on the application of thyroid hormones in hPSC culture medium. The authors declare that they have no other competing interests.
Figures

Similar articles
-
Crotonylation of GAPDH regulates human embryonic stem cell endodermal lineage differentiation and metabolic switch.Stem Cell Res Ther. 2023 Apr 3;14(1):63. doi: 10.1186/s13287-023-03290-y. Stem Cell Res Ther. 2023. PMID: 37013624 Free PMC article.
-
Effect of fibronectin, FGF-2, and BMP4 in the stemness maintenance of BMSCs and the metabolic and proteomic cues involved.Stem Cell Res Ther. 2021 Mar 6;12(1):165. doi: 10.1186/s13287-021-02227-7. Stem Cell Res Ther. 2021. PMID: 33676544 Free PMC article.
-
Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRα1.Stem Cells Dev. 2012 Sep 20;21(14):2667-81. doi: 10.1089/scd.2012.0023. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468949 Free PMC article.
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
-
Lnc-ing pluripotency maintenance and early differentiation in human pluripotent stem cells.FASEB J. 2021 Apr;35(4):e21438. doi: 10.1096/fj.202002278R. FASEB J. 2021. PMID: 33749897 Review.
Cited by
-
Comparison on the consistency of Mindray and Siemens chemiluminescence analyzers for detecting FT3, FT4 and TSH in patients with hyper- and hypothyroidism.Ann Transl Med. 2022 Oct;10(20):1133. doi: 10.21037/atm-22-4589. Ann Transl Med. 2022. PMID: 36388840 Free PMC article.
-
The thyroid hormone enhances mouse embryonic fibroblasts reprogramming to pluripotent stem cells: role of the nuclear receptor corepressor 1.Front Endocrinol (Lausanne). 2023 Dec 1;14:1235614. doi: 10.3389/fendo.2023.1235614. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38107517 Free PMC article.
-
ERK5 promotes autocrine expression to sustain mitogenic balance for cell fate specification in human pluripotent stem cells.Stem Cell Reports. 2024 Sep 10;19(9):1320-1335. doi: 10.1016/j.stemcr.2024.07.007. Epub 2024 Aug 15. Stem Cell Reports. 2024. PMID: 39151429 Free PMC article.
-
Thyroid hormone receptor α1: a novel regulator of thyroid cancer cell differentiation.Oncogene. 2023 Oct;42(41):3075-3086. doi: 10.1038/s41388-023-02815-2. Epub 2023 Aug 26. Oncogene. 2023. PMID: 37634007 Free PMC article.
-
VEGF Expression in Umbilical Cord MSC Depends on the Patient's Health, the Week of Pregnancy in Which the Delivery Took Place, and the Body Weight of the Newborn - Preliminary Report.Stem Cells Cloning. 2023 Apr 27;16:5-18. doi: 10.2147/SCCAA.S399303. eCollection 2023. Stem Cells Cloning. 2023. PMID: 37139466 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

