Adipose stem cell-derived extracellular vesicles ameliorates corticosterone-induced apoptosis in the cortical neurons via inhibition of ER stress
- PMID: 35313975
- PMCID: PMC8935810
- DOI: 10.1186/s13287-022-02785-4
Adipose stem cell-derived extracellular vesicles ameliorates corticosterone-induced apoptosis in the cortical neurons via inhibition of ER stress
Abstract
Background: Corticosterone (CORT) can induce neuronal damage in various brain regions, including the cerebral cortex, the region implicated in depression. However, the underlying mechanisms of these CORT-induced effects remain poorly understood. Recently, many studies have suggested that adipose stem cell-derived extracellular vesicles (A-EVs) protect neurons in the brain.
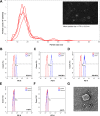
Methods: To investigated neuroprotection effects of A-EVs in the CORT-induced cortical neurons, we cultured cortical neurons from E15 mice for 7 days, and the cultured cortical neurons were pretreated with different numbers (5 × 105-107 per mL) of A-EVs (A-EVs5, A-EVs6, A-EVs7) for 30 min followed by administration of 200 μM CORT for 24 h.
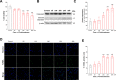
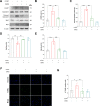
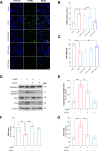
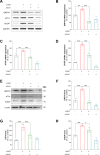
Results: Here, we show that A-EVs exert antiapoptotic effects by inhibiting endoplasmic reticulum (ER) stress in CORT-induced cortical neurons. We found that A-EVs prevented neuronal cell death induced by CORT in cultured cortical neurons. More importantly, we found that CORT exposure in cortical neurons resulted in increased levels of apoptosis-related proteins such as cleaved caspase-3. However, pretreatment with A-EVs rescued the levels of caspase-3. Intriguingly, CORT-induced apoptosis involved upstream activation of ER stress proteins such as GRP78, CHOP and ATF4. However, pretreatment with A-EVs inhibited ER stress-related protein expression.
Conclusion: Our findings reveal that A-EVs exert antiapoptotic effects via inhibition of ER stress in CORT-induced cell death.
Keywords: Adipose stem cell-derived extracellular vesicles (A-EVs); Apoptosis; Cortical neurons; Corticosterone; ER stress.
© 2022. The Author(s).
Conflict of interest statement
Authors declare no competing interests.
Figures
Similar articles
-
Extracellular Vesicles from Neural Stem Cells Carry microRNA-16-5p to Reduce Corticosterone-induced Neuronal Injury in Depression Rats.Neuroscience. 2024 Feb 6;538:95-109. doi: 10.1016/j.neuroscience.2023.09.016. Epub 2023 Sep 29. Neuroscience. 2024. PMID: 37778691
-
Alpha-lipoic acid protects against cadmium-induced neuronal injury by inhibiting the endoplasmic reticulum stress eIF2α-ATF4 pathway in rat cortical neurons in vitro and in vivo.Toxicology. 2019 Feb 15;414:1-13. doi: 10.1016/j.tox.2018.12.005. Epub 2018 Dec 31. Toxicology. 2019. PMID: 30605698
-
Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stromal Cells Protect Cardiac Cells Against Hypoxia/Reoxygenation Injury by Inhibiting Endoplasmic Reticulum Stress via Activation of the PI3K/Akt Pathway.Cell Transplant. 2020 Jan-Dec;29:963689720945677. doi: 10.1177/0963689720945677. Cell Transplant. 2020. PMID: 32864999 Free PMC article.
-
Butylparaben Induces the Neuronal Death Through the ER Stress-Mediated Apoptosis of Primary Cortical Neurons.Neurotox Res. 2022 Feb;40(1):36-43. doi: 10.1007/s12640-021-00452-9. Epub 2022 Jan 4. Neurotox Res. 2022. PMID: 34981454
-
Multiple roles of neuronal extracellular vesicles in neurological disorders.Front Cell Neurosci. 2022 Sep 20;16:979856. doi: 10.3389/fncel.2022.979856. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36204449 Free PMC article. Review.
Cited by
-
Premeiotic deletion of Eif2s2 causes oocyte arrest at the early diplotene stage and apoptosis in mice.Cell Prolif. 2024 Dec;57(12):e13718. doi: 10.1111/cpr.13718. Epub 2024 Jul 24. Cell Prolif. 2024. PMID: 39044637 Free PMC article.
-
Research progress in role of exosomes exosomes in mental disorders.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 May 28;48(5):771-781. doi: 10.11817/j.issn.1672-7347.2023.220379. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37539580 Free PMC article. Chinese, English.
-
Acid-responsive CST@NPs enhanced diabetic wound healing through rescuing mitochondrial dysfunction.Bioact Mater. 2024 Oct 23;44:269-282. doi: 10.1016/j.bioactmat.2024.10.004. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39507372 Free PMC article.
-
Update on the roles and applications of extracellular vesicles in depression.World J Psychiatry. 2025 Mar 19;15(3):102643. doi: 10.5498/wjp.v15.i3.102643. eCollection 2025 Mar 19. World J Psychiatry. 2025. PMID: 40110012 Free PMC article. Review.
-
Synovial Fluid-Derived Extracellular Vesicles of Patients with Arthritides Contribute to Hippocampal Synaptic Dysfunctions and Increase with Mood Disorders Severity in Humans.Cells. 2022 Jul 23;11(15):2276. doi: 10.3390/cells11152276. Cells. 2022. PMID: 35892573 Free PMC article.
References
-
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteom. 2009;6:267–283. - PubMed
-
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. - PubMed
-
- Christianson HC, Svensson KJ, Belting M. Exosome and microvesicle mediated phene transfer in mammalian cells. Semin Cancer Biol. 2014;28:31–38. - PubMed
-
- Ela S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

