Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: a systematic review and meta-analysis
- PMID: 35313985
- PMCID: PMC8935249
- DOI: 10.1186/s13287-022-02786-3
Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: a systematic review and meta-analysis
Abstract
Background: Systemic sclerosis (SSc) is an autoimmune disease with high morbidity and mortality characterized by fibrosis of the skin and internal organs. Some studies have investigated the use of stem cells to treat SSc. Herein, a systematic review and meta-analysis was conducted to determine the efficacy and safety of mesenchymal stem cells (MSCs) in the treatment of SSc.
Methods: PubMed, Embase, Cochrane Library, Web of Science, OVID, China National Knowledge Infrastructure and Wanfang databases were searched up to February 1, 2021. Literature screening, data extraction and quality assessment were conducted independently by two researchers in according to the inclusion and exclusion criteria. The discrepancies were resolved by a third researcher.
Results: A total of 9 studies encompassing 133 SSc patients were included in the study. Compared to the baseline after treatment with MSCs: 1. The modified Rodnan skin score (mRSS) was significantly reduced in patients with SSc (P < 0.00001). 2. MSCs decreased the number of digital ulcer, mouth handicap scale, and visual analog scale of hand pain in SSc patients (P = 0.0007 and P = 0.03, respectively). 3. No statistical differences were detected in Raynaud's condition score and Cochin hand function scale score at 6 months of MSCs therapy (P = 0.5 and P = 0.62). 4. After 12 months of follow-up, MSCs improve carbon monoxide diffusing capacity and forced vital capacity of SSc patients (P < 0.05). 5. Overall, MSCs application was safe; a few cases exhibited swelling at the injection site, diarrhea and arthralgia, which had self-recovery, and no severe adverse events occurred in the included trials.
Conclusions: MSC therapy improves the degree of skin thickening, lung function, and mouth opening and relieves finger ulcers and pain in patients with SSc without severe adverse events. Thus, MSCs or MSCs combined with plasma and traditional medicine might be an effective and promising treatment of SSc patients. PROSPERO registration number: CRD42020200350.
Keywords: Mesenchymal stem cells; Meta-analysis; Systemic sclerosis; Treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



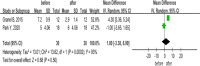





References
-
- Friedenstein A, Gorskaja J, Kulagina N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

