A local QSAR model based on the stability of nitrenium ions to support the ICH M7 expert review on the mutagenicity of primary aromatic amines
- PMID: 35313995
- PMCID: PMC8935809
- DOI: 10.1186/s41021-022-00238-1
A local QSAR model based on the stability of nitrenium ions to support the ICH M7 expert review on the mutagenicity of primary aromatic amines
Abstract
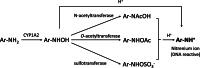
Background: Aromatic amines, often used as intermediates for pharmaceutical synthesis, may be mutagenic and therefore pose a challenge as metabolites or impurities in drug development. However, predicting the mutagenicity of aromatic amines using commercially available, quantitative structure-activity relationship (QSAR) tools is difficult and often requires expert review. In this study, we developed a shareable QSAR tool based on nitrenium ion stability.
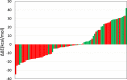
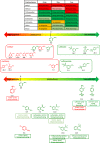
Results: The evaluation using in-house aromatic amine intermediates revealed that our model has prediction accuracy of aromatic amine mutagenicity comparable to that of commercial QSAR tools. The effect of changing the number and position of substituents on the mutagenicity of aromatic amines was successfully explained by the change in the nitrenium ion stability. Furthermore, case studies showed that our QSAR tool can support the expert review with quantitative indicators.
Conclusions: This local QSAR tool will be useful as a quantitative support tool to explain the substituent effects on the mutagenicity of primary aromatic amines. By further refinement through method sharing and standardization, our tool can support the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M7 expert review with quantitative indicators.
Keywords: Expert review; ICH M7; In silico; Nitrenium ion; Primary aromatic amine; Quantitative structure–activity relationship (QSAR).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- ICH. ICH harmonized guideline. Assessment and control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Current Step 4 version dated 31 March 2017. https://www.ich.org/home.html. Accessed 31 Jan 2022.
-
- Honma M, Kitazawa A, Cayley A, Williams RV, Barber C, Hanser T, Saiakhov R, Chakravarti S, Myatt GJ, Cross KP, Benfenati E, Raitano G, Mekenyan O, Petkov P, Bossa C, Benigni R, Battistelli CL, Giuliani A, Tcheremenskaia O, DeMeo C, Norinder U, Koga H, Jose C, Jeliazkova N, Kochev N, Paskaleva V, Yang C, Daga PR, Clark RD, Rathman J. Improvement of quantitative structure–activity relationship (QSAR) tools for predicting Ames mutagenicity: outcomes of the Ames/QSAR international challenge project. Mutagenesis. 2019;34(1):3–16. doi: 10.1093/mutage/gey031. - DOI - PMC - PubMed
-
- Hasselgren C, Bercu J, Cayley A, Cross K, Glowienke S, Kruhlak N, Muster W, Nicolette J, Reddy MV, Saiakhov R, Dobo K. Management of pharmaceutical ICH M7 (Q)SAR predictions – the impact of model updates. Regul Toxicol Pharmacol. 2020;118:104807. doi: 10.1016/j.yrtph.2020.104807. - DOI - PMC - PubMed
-
- Ahlberg E, Amberg A, Beilke LD, Bower D, Cross KP, Custer L, Ford KA, van Gompel J, Harvey J, Honma M, Jolly R, Joossens E, Kemper RA, Kenyon M, Kruhlak N, Kuhnke L, Leavitt P, Naven R, Neilan C, Quigley DP, Shuey D, Spirkl HP, Stavitskaya L, Teasdale A, White A, Wichard J, Zwickl C, Myatt GJ. Extending (Q)SARs to incorporate proprietary knowledge for regulatory purposes: a case study using aromatic amine mutagenicity. Regul Toxicol Pharmacol. 2016;77:1–12. doi: 10.1016/j.yrtph.2016.02.003. - DOI - PubMed
-
- Patel M, Kranz M, Munoz-Muriedas J, Harvey JS, Giddings A, Swallow S, Fellows M, Naven R, Werner AL, Yeo DJ, Bringezu F, Wichard J, Sutter A, Glowienke S, Whitehead L, Selby M, Reuberson J, Atienzar F, Gerets H, Kenyon MO, Dobo KL, Walter MW, Jolly RA, Amberg A, Spirkl HP, Muster W, van Gompel J. A pharma-wide approach to address the genotoxicity prediction of primary aromatic amines. Comput Toxicol. 2018;7:27–35. doi: 10.1016/j.comtox.2018.06.002. - DOI
LinkOut - more resources
Full Text Sources

