Mycobacterium tuberculosis encodes a YhhN family membrane protein with lysoplasmalogenase activity that protects against toxic host lysolipids
- PMID: 35314194
- PMCID: PMC9052158
- DOI: 10.1016/j.jbc.2022.101849
Mycobacterium tuberculosis encodes a YhhN family membrane protein with lysoplasmalogenase activity that protects against toxic host lysolipids
Abstract
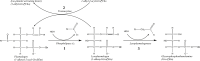
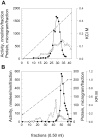
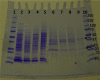
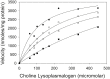
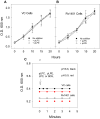
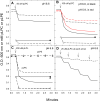
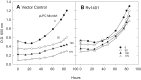
The pathogen Mycobacterium tuberculosis (M.tb) resides in human macrophages, wherein it exploits host lipids for survival. However, little is known about the interaction between M.tb and macrophage plasmalogens, a subclass of glycerophospholipids with a vinyl ether bond at the sn-1 position of the glycerol backbone. Lysoplasmalogens, produced from plasmalogens by hydrolysis at the sn-2 carbon by phospholipase A2, are potentially toxic but can be broken down by host lysoplasmalogenase, an integral membrane protein of the YhhN family that hydrolyzes the vinyl ether bond to release a fatty aldehyde and glycerophospho-ethanolamine or glycerophospho-choline. Curiously, M.tb encodes its own YhhN protein (MtbYhhN), despite having no endogenous plasmalogens. To understand the purpose of this protein, the gene for MtbYhhN (Rv1401) was cloned and expressed in Mycobacterium smegmatis (M.smeg). We found the partially purified protein exhibited abundant lysoplasmalogenase activity specific for lysoplasmenylethanolamine or lysoplasmenylcholine (pLPC) (Vmax∼15.5 μmol/min/mg; Km∼83 μM). Based on cell density, we determined that lysoplasmenylethanolamine, pLPC, lysophosphatidylcholine, and lysophosphatidylethanolamine were not toxic to M.smeg cells, but pLPC and LPC were highly toxic to M.smeg spheroplasts, which are cell wall-deficient mycobacterial forms. Importantly, spheroplasts prepared from M.smeg cells overexpressing MtbYhhN were protected from membrane disruption/lysis by pLPC, which was rapidly depleted from the media. Finally, we found that overexpression of full-length MtbYhhN in M.smeg increased its survival within human macrophages by 2.6-fold compared to vector controls. These data support the hypothesis that MtbYhhN protein confers a growth advantage for mycobacteria in macrophages by cleaving toxic host pLPC into potentially energy-producing products.
Keywords: Mycobacterium smegmatis; Mycobacterium tuberculosis Rv1401 gene; YhhN protein; cell injury and protection; cell wall–deficient; lysoplasmalogen/lysophospholipid; lysoplasmalogenase; macrophage; phospholipase A; spheroplasts.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
The YhhN protein of Legionella pneumophila is a Lysoplasmalogenase.Biochim Biophys Acta. 2015 Feb;1848(2):742-51. doi: 10.1016/j.bbamem.2014.11.011. Epub 2014 Nov 16. Biochim Biophys Acta. 2015. PMID: 25445671 Free PMC article.
-
Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen.J Biol Chem. 2011 Jul 15;286(28):24916-30. doi: 10.1074/jbc.M111.247163. Epub 2011 Apr 22. J Biol Chem. 2011. PMID: 21515882 Free PMC article.
-
Functional characterization of TMEM86A and TMEM86B mutants by a novel lysoplasmalogenase assay.J Lipid Res. 2025 Apr;66(4):100766. doi: 10.1016/j.jlr.2025.100766. Epub 2025 Feb 28. J Lipid Res. 2025. PMID: 40024572 Free PMC article.
-
Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue.J Mol Neurosci. 2001 Apr-Jun;16(2-3):263-72; discussion 279-84. doi: 10.1385/jmn:16:2-3:263. J Mol Neurosci. 2001. PMID: 11478381 Review.
-
PE_PGRS: Vital proteins in promoting mycobacterial survival and modulating host immunity and metabolism.Cell Microbiol. 2021 Mar;23(3):e13290. doi: 10.1111/cmi.13290. Epub 2020 Dec 1. Cell Microbiol. 2021. PMID: 33217152 Review.
Cited by
-
Regulation of plasmalogen metabolism and traffic in mammals: The fog begins to lift.Front Cell Dev Biol. 2022 Aug 31;10:946393. doi: 10.3389/fcell.2022.946393. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120579 Free PMC article. Review.
References
-
- Rohde K., Yates R.M., Purdy G.E., Russell D.G. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 2007;210:37–54. - PubMed
-
- Sturgill-Koszycki S., Schlesinger P.H., Chakraborty P., Haddix P.L., Collins H.L., Fok A.K., Allen R.D., G luck S.L., Heuser J., Russell D.G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Nature. 2004;263:678–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

