Safety, efficacy and delivery of multiple nucleoside analogs via drug encapsulated carbon (DECON) based sustained drug release platform
- PMID: 35314347
- PMCID: PMC9113733
- DOI: 10.1016/j.ejpb.2022.03.001
Safety, efficacy and delivery of multiple nucleoside analogs via drug encapsulated carbon (DECON) based sustained drug release platform
Abstract
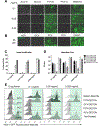
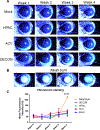
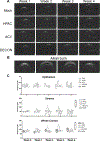
Acyclovir and similar nucleoside analogs form an essential frontline treatment for various herpesvirus infections of the eye. However, these drugs have low ocular retention when delivered topically and need to be administered several times every day. We have previously demonstrated that acyclovir loaded into activated carbon can be used to significantly decrease dosage frequency in a murine model of ocular infection. In this study, we demonstrate that other nucleoside analogs such as ganciclovir, penciclovir and famciclovir have excellent loading and release profile similar to acyclovir. Similarly we also demonstrate that nucleoside analog loaded carbons termed DECON are effective at very low concentrations in treating active viral infection of human corneal epithelial cells. In this study, using a variety of techniques to evaluate corneal dryness, nerve sensitivity, intraocular pressure, corneal thickness, and somatic inflammation, we report that DECON is well tolerated after administration three times daily over the course of four weeks.
Keywords: Activated carbon; Acyclovir; Herpes simplex virus; Nucleoside analogs; Ocular toxicity; Topical delivery.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

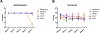
Similar articles
-
Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery.Sci Adv. 2019 Aug 14;5(8):eaax0780. doi: 10.1126/sciadv.aax0780. eCollection 2019 Aug. Sci Adv. 2019. PMID: 31453334 Free PMC article.
-
Penciclovir cream--improved topical treatment for herpes simplex infections.Skin Pharmacol Physiol. 2004 Sep-Oct;17(5):214-8. doi: 10.1159/000080214. Skin Pharmacol Physiol. 2004. PMID: 15452407 Review.
-
A comparative study to evaluate the efficacy and safety of acyclovir and famciclovir in the management of herpes zoster.J Clin Diagn Res. 2013 Dec;7(12):2904-7. doi: 10.7860/JCDR/2013/7884.3670. Epub 2013 Nov 18. J Clin Diagn Res. 2013. PMID: 24551671 Free PMC article.
-
Lack of effect of treatment with penciclovir or acyclovir on the establishment of latent HSV-1 in primary sensory neurons in culture.Antiviral Res. 2001 Oct;52(1):19-24. doi: 10.1016/s0166-3542(01)00155-3. Antiviral Res. 2001. PMID: 11530184
-
Acyclic nucleosides as antiviral compounds.Mol Biotechnol. 1996 Apr;5(2):125-37. doi: 10.1007/BF02789061. Mol Biotechnol. 1996. PMID: 8734425 Review.
Cited by
-
The Interplay of Genital Herpes with Cellular Processes: A Pathogenesis and Therapeutic Perspective.Viruses. 2023 Oct 31;15(11):2195. doi: 10.3390/v15112195. Viruses. 2023. PMID: 38005873 Free PMC article. Review.
References
-
- Tsatsos M et al. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents – comment. Clin. Exp. Ophthalmol. 45, 932 (2017). - PubMed
-
- Lairson DR, Begley CE, Reynolds TF & Wilhelmus KR Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch. Ophthalmol. 121, 108–112 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

