Effects of Hydrochlorothiazide and Metformin on Aquaresis and Nephroprotection by a Vasopressin V2 Receptor Antagonist in ADPKD: A Randomized Crossover Trial
- PMID: 35314480
- PMCID: PMC8993480
- DOI: 10.2215/CJN.11260821
Effects of Hydrochlorothiazide and Metformin on Aquaresis and Nephroprotection by a Vasopressin V2 Receptor Antagonist in ADPKD: A Randomized Crossover Trial
Abstract
Background and objectives: The vasopressin V2 receptor antagonist tolvaptan is the only drug that has been proven to be nephroprotective in autosomal dominant polycystic kidney disease (ADPKD). Tolvaptan also causes polyuria, limiting tolerability. We hypothesized that cotreatment with hydrochlorothiazide or metformin may ameliorate this side effect.
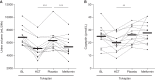
Design, setting, participants, & measurements: We performed a clinical study and an animal study. In a randomized, controlled, double-blind, crossover trial, we included 13 tolvaptan-treated patients with ADPKD. Patients were treated for three 2-week periods with hydrochlorothiazide, metformin, or placebo in random order. Primary outcome was change in 24-hour urine volume. We also measured GFR and a range of metabolic and kidney injury markers.
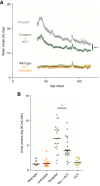
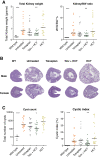
Results: Patients (age 45±8 years, 54% women, measured GFR of 55±11 ml/min per 1.73 m2) had a baseline urine volume on tolvaptan of 6.9±1.4 L/24 h. Urine volume decreased to 5.1 L/24 h (P<0.001) with hydrochlorothiazide and to 5.4 L/24 h (P<0.001) on metformin. During hydrochlorothiazide treatment, plasma copeptin (surrogate for vasopressin) decreased, quality of life improved, and several markers of kidney damage and glucose metabolism improved. Metformin did not induce changes in these markers or in quality of life. Given these results, the effect of adding hydrochlorothiazide to tolvaptan was investigated on long-term kidney outcome in an animal experiment. Water intake in tolvaptan-hydrochlorothiazide cotreated mice was 35% lower than in mice treated with tolvaptan only. Combination treatment was superior to "no treatment" on markers of disease progression (kidney weight, P=0.003 and cystic index, P=0.04) and superior or equal to tolvaptan alone.
Conclusions: Both metformin and hydrochlorothiazide reduced tolvaptan-caused polyuria in a short-term study. Hydrochlorothiazide also reduced polyuria in a long-term animal model without negatively affecting nephroprotection.
Podcast: This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_03_21_CJN11260821.mp3.
Keywords: ADPKD; clinical trial; diabetes insipidus; diuretics; hydrochlorothiazide; metformin; receptors; vasopressin.
Copyright © 2022 by the American Society of Nephrology.
Figures


References
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 - PubMed
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 - PubMed
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 - PubMed
-
- Blair HA, Keating GM: Tolvaptan: A review in autosomal dominant polycystic kidney disease. Drugs 75: 1797–1806, 2015 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

