Spectrum of Phenotypic, Genetic, and Functional Characteristics in Patients With Epilepsy With KCNC2 Pathogenic Variants
- PMID: 35314505
- PMCID: PMC9162046
- DOI: 10.1212/WNL.0000000000200660
Spectrum of Phenotypic, Genetic, and Functional Characteristics in Patients With Epilepsy With KCNC2 Pathogenic Variants
Abstract
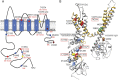
Background and objectives: KCNC2 encodes Kv3.2, a member of the Shaw-related (Kv3) voltage-gated potassium channel subfamily, which is important for sustained high-frequency firing and optimized energy efficiency of action potentials in the brain. The objective of this study was to analyze the clinical phenotype, genetic background, and biophysical function of disease-associated Kv3.2 variants.
Methods: Individuals with KCNC2 variants detected by exome sequencing were selected for clinical, further genetic, and functional analysis. Cases were referred through clinical and research collaborations. Selected de novo variants were examined electrophysiologically in Xenopus laevis oocytes.
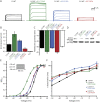
Results: We identified novel KCNC2 variants in 18 patients with various forms of epilepsy, including genetic generalized epilepsy (GGE), developmental and epileptic encephalopathy (DEE) including early-onset absence epilepsy, focal epilepsy, and myoclonic-atonic epilepsy. Of the 18 variants, 10 were de novo and 8 were classified as modifying variants. Eight drug-responsive patients became seizure-free using valproic acid as monotherapy or in combination, including severe DEE cases. Functional analysis of 4 variants demonstrated gain of function in 3 severely affected DEE cases and loss of function in 1 case with a milder phenotype (GGE) as the underlying pathomechanisms.
Discussion: These findings implicate KCNC2 as a novel causative gene for epilepsy and emphasize the critical role of KV3.2 in the regulation of brain excitability.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- World Health Organization. WHO Epilepsy: A Public Health Imperative. World Health Organization; 2019.
-
- Weber YG, Biskup S, Helbig KL, Von Spiczak S, Lerche H. The role of genetic testing in epilepsy diagnosis and management. Expert Rev Mol Diagn. 2017;17(8):739-750. - PubMed
-
- Butler A, Wei AG, Baker K, Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989;243(4893):943-947. - PubMed
-
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24(9):517-526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
