2, 3, 5, 4'-tetrahydroxystilbene-2-O-beta-D-glucoside protects against neuronal cell death and traumatic brain injury-induced pathophysiology
- PMID: 35314517
- PMCID: PMC9004580
- DOI: 10.18632/aging.203958
2, 3, 5, 4'-tetrahydroxystilbene-2-O-beta-D-glucoside protects against neuronal cell death and traumatic brain injury-induced pathophysiology
Abstract
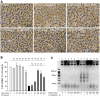
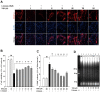
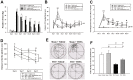
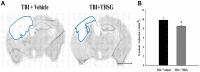
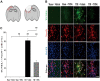
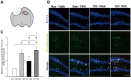
Traumatic brain injury (TBI) is a global health issue that affects at least 10 million people per year. Neuronal cell death and brain injury after TBI, including apoptosis, inflammation, and excitotoxicity, have led to detrimental effects in TBI. 2, 3, 5, 4'-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG), a water-soluble compound extracted from the Chinese herb Polygonum multiflorum, has been shown to exert various biological functions. However, the effects of THSG on TBI is still poorly understood. THSG reduced L-glutamate-induced DNA fragmentation and protected glial and neuronal cell death after L-glutamate stimulation. Our results also showed that TBI caused significant behavioral deficits in the performance of beam walking, mNSS, and Morris water maze tasks in a mouse model. Importantly, daily administration of THSG (60 mg/kg/day) after TBI for 21 days attenuated the injury severity score, promoted motor coordination, and improved cognitive performance post-TBI. Moreover, administration of THSG also dramatically decreased the brain lesion volume. THSG reduced TBI-induced neuronal apoptosis in the brain cortex 24 h after TBI. Furthermore, THSG increased the number of immature neurons in the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus. Our results demonstrate that THSG exerts neuroprotective effects on glutamate-induced excitotoxicity and glial and neuronal cell death. The present study also demonstrated that THSG effectively protects against TBI-associated motor and cognitive impairment, at least in part, by inhibiting TBI-induced apoptosis and promoting neurogenesis.
Keywords: Chinese herb; apoptosis; cognitive dysfunction; excitotoxicity; traumatic brain injury.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

