Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion
- PMID: 35314680
- PMCID: PMC8938495
- DOI: 10.1038/s41467-022-29137-3
Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion
Abstract
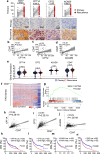
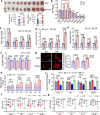
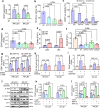
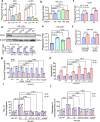
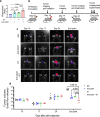
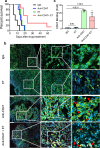
Glioblastoma multiforme (GBM) remains the top challenge to radiotherapy with only 25% one-year survival after diagnosis. Here, we reveal that co-enhancement of mitochondrial fatty acid oxidation (FAO) enzymes (CPT1A, CPT2 and ACAD9) and immune checkpoint CD47 is dominant in recurrent GBM patients with poor prognosis. A glycolysis-to-FAO metabolic rewiring is associated with CD47 anti-phagocytosis in radioresistant GBM cells and regrown GBM after radiation in syngeneic mice. Inhibition of FAO by CPT1 inhibitor etomoxir or CRISPR-generated CPT1A-/-, CPT2-/-, ACAD9-/- cells demonstrate that FAO-derived acetyl-CoA upregulates CD47 transcription via NF-κB/RelA acetylation. Blocking FAO impairs tumor growth and reduces CD47 anti-phagocytosis. Etomoxir combined with anti-CD47 antibody synergizes radiation control of regrown tumors with boosted macrophage phagocytosis. These results demonstrate that enhanced fat acid metabolism promotes aggressive growth of GBM with CD47-mediated immune evasion. The FAO-CD47 axis may be targeted to improve GBM control by eliminating the radioresistant phagocytosis-proofing tumor cells in GBM radioimmunotherapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures