Inorganic phosphate in growing calcium carbonate abalone shell suggests a shared mineral ancestral precursor
- PMID: 35314701
- PMCID: PMC8938516
- DOI: 10.1038/s41467-022-29169-9
Inorganic phosphate in growing calcium carbonate abalone shell suggests a shared mineral ancestral precursor
Abstract
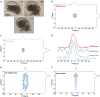
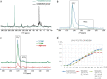
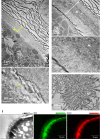
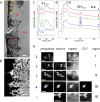
The presence of phosphate from different origins (inorganic, bioorganic) is found more and more in calcium carbonate-based biominerals. Phosphate is often described as being responsible for the stabilization of the transient amorphous calcium carbonate phase. In order to specify the composition of the mineral phase deposited at the onset of carbonated shell formation, the present study investigates, down to the nanoscale, the growing shell from the European abalone Haliotis tuberculata, using a combination of solid state nuclear magnetic resonance, scanning transmission electron microscope and spatially-resolved electron energy loss spectroscopy techniques. We show the co-occurrence of inorganic phosphate with calcium and carbonate throughout the early stages of abalone shell formation. One possible hypothesis is that this first-formed mixed mineral phase represents the vestige of a shared ancestral mineral precursor that appeared early during Evolution. In addition, our findings strengthen the idea that the final crystalline phase (calcium carbonate or phosphate) depends strongly on the nature of the mineral-associated proteins in vivo.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification.J Struct Biol. 2015 Jun;190(3):291-303. doi: 10.1016/j.jsb.2015.04.014. Epub 2015 Apr 28. J Struct Biol. 2015. PMID: 25934395
-
Ultrastructure, chemistry and mineralogy of the growing shell of the European abalone Haliotis tuberculata.J Struct Biol. 2010 Sep;171(3):277-90. doi: 10.1016/j.jsb.2010.05.012. Epub 2010 May 27. J Struct Biol. 2010. PMID: 20553887
-
Amorphous Ca-phosphate precursors for Ca-carbonate biominerals mediated by Chromohalobacter marismortui.ISME J. 2010 Jul;4(7):922-32. doi: 10.1038/ismej.2010.17. Epub 2010 Feb 25. ISME J. 2010. PMID: 20182524
-
Revisiting the physical and chemical nature of the mineral component of bone.Acta Biomater. 2025 Apr;196:1-16. doi: 10.1016/j.actbio.2025.01.055. Epub 2025 Jan 30. Acta Biomater. 2025. PMID: 39892685 Review.
-
Calcium carbonate polyamorphism and its role in biomineralization: how many amorphous calcium carbonates are there?Angew Chem Int Ed Engl. 2012 Nov 26;51(48):11960-70. doi: 10.1002/anie.201203125. Epub 2012 Nov 4. Angew Chem Int Ed Engl. 2012. PMID: 23124964 Review.
Cited by
-
Amorphous Calcium Phosphate and Amorphous Calcium Phosphate Carboxylate: Synthesis and Characterization.ACS Omega. 2023 Jul 17;8(30):26782-26792. doi: 10.1021/acsomega.3c00796. eCollection 2023 Aug 1. ACS Omega. 2023. PMID: 37546623 Free PMC article.
-
Phosphate ion removal from aqueous solution using snail shell dust: biosorption potential of waste shells of edible snails.RSC Adv. 2022 Oct 25;12(46):30011-30023. doi: 10.1039/d2ra03852h. eCollection 2022 Oct 17. RSC Adv. 2022. PMID: 36329945 Free PMC article.
-
The contribution of amyloid deposition in the aortic valve to calcification and aortic stenosis.Nat Rev Cardiol. 2023 Jun;20(6):418-428. doi: 10.1038/s41569-022-00818-2. Epub 2023 Jan 9. Nat Rev Cardiol. 2023. PMID: 36624274 Free PMC article. Review.
-
Effect of Dietary Insect Meal and Grape Marc Inclusion on Flavor Volatile Compounds and Shell Color of Juvenile Abalone Haliotis iris.Aquac Nutr. 2023 Jul 18;2023:6628232. doi: 10.1155/2023/6628232. eCollection 2023. Aquac Nutr. 2023. PMID: 37496745 Free PMC article.
-
Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization.Int J Mol Sci. 2024 Mar 13;25(6):3261. doi: 10.3390/ijms25063261. Int J Mol Sci. 2024. PMID: 38542235 Free PMC article. Review.
References
-
- Lowenstam H. Minerals formed by organisms. Science. 1981;211:1126–1131. - PubMed
-
- Lowenstam, H. A. & S. Weiner. On Biomineralization (Oxford University Press, 1989).
-
- Baran, E. J. Review: Natural oxalates and their analogous synthetic complexes. J. Coord. Chem.67, 3734–3768 (2014).
-
- Addadi L, Raz S, Weiner S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003;15:959–970.
-
- Taylor MG, Simkiss K, Greaves GN, Okazaki M, Mann S. An X-ray absorption spectroscopy study of the structure and transformation of amorphous calcium carbonate from plant cystoliths. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 1993;252:75–80.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

