Perceptions of pharmacists on the quality of automated blood pressure devices: a national survey
- PMID: 35314763
- PMCID: PMC9995266
- DOI: 10.1038/s41371-022-00670-4
Perceptions of pharmacists on the quality of automated blood pressure devices: a national survey
Abstract
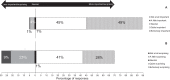
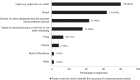
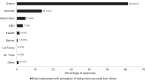
A recent study found that only 23.8% of blood pressure (BP) devices available for purchase from Australian pharmacies were validated for accuracy. The extent to which pharmacists are aware of this, and other issues related to the accuracy of BP devices, is not known and gathering this information was the aim of this study. An online survey of Australian pharmacists was distributed via the Pharmaceutical Society of Australia between 1 October and 25 November 2020. Questions were focused on the views of pharmacists related to the accuracy of BP devices. Two hundred and ten pharmacists completed the survey. The accuracy of BP devices sold by pharmacists was considered 'quite' or 'extremely important' to most respondents (94%). However, most respondents (90%) were unaware that less than one-quarter of BP devices sold by Australian pharmacies were validated, and this was 'quite' or 'extremely surprising' to many (69%). Many respondents (64%) associated a particular brand of BP device with greater accuracy. There was low awareness on proper ways to identify accurate BP devices, such as checking reputable online databases (43%). BP devices were stocked in respondents' pharmacies based on perceived quality (50%), accuracy (40%), or as determined by the pharmacy chain (36%). In conclusion, providing accurate BP devices to consumers is important to pharmacists, but they were generally unaware that most devices available from pharmacies were not validated for accuracy. Pharmacist education, alongside advocacy for policies including regulations and strategic action, is required to ensure only validated BP devices are sold in Australia.
© 2022. The Author(s).
Conflict of interest statement
DSP and JES are consultants of HEARTS in the Americas, an initiative of the Pan American Health Organisation. AES has received speaker honoraria from Omron Healthcare. NRCC reports personal fees from Resolve to Save Lives (RTSL) and the World Bank, and is an unpaid consultant on dietary sodium and hypertension control to numerous governmental and non-governmental organisations. RP is Canadian representative to the ISO Sphygmomanometer committee and sits on the AAMI Sphygmomanometer committee and Co-Founder and CEO of a digital health company (mmHg Inc), based at the University of Alberta. JES is principal investigator of a National Health and Medical Research Council partnership grant (S0026615) that includes a medical technology company that manufactures a central BP monitor.
Figures
Comment in
-
Pharmacists' knowledge of automated blood pressure devices.J Hum Hypertens. 2022 Nov;36(11):1027-1028. doi: 10.1038/s41371-022-00746-1. Epub 2022 Aug 26. J Hum Hypertens. 2022. PMID: 36028751 No abstract available.

